The review provides information related to the inspection of manufacturers of medicinal products for veterinary use for compliance with the requirements of the Good Manufacturing Practice (GMP) which is carried out by specialists of the Federal State Budgetary Institution “The All-Russian State Center for Quality and Standardization of Veterinary Drugs and Feed” (FSBI “VGNKI”) subordinated to Rosselkhoznadzor.
Inspection results
According to the register of issued conclusions published on the Rosselkhoznadzor website 20.06.2022, in the first half of 2022, foreign manufacturers were issued 3 conclusions on compliance with the GMP Rules requirements [1]. The first of these conclusions was made for a site located in Spain and two of the others for sites located in Korea and Croatia.
The largest number of Russian inspections, based on the results of which, over the past 5 years, decisions were made to issue/refuse to issue a GMP-conclusion (23, which is 17%), was conducted at manufacturing sites located in France.
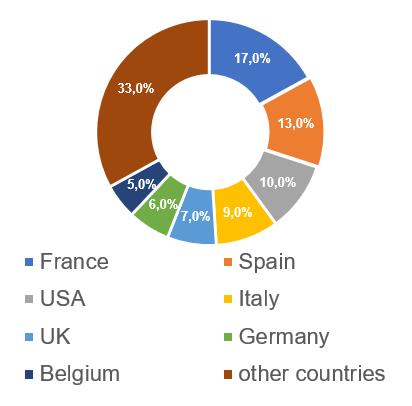
Information about French manufacturers of veterinary medicines and their inspection for compliance with the GMP Rules requirements is presented here: https://pharmprom.ru/en/french-manufacturers-of-veterinary-medicines-and-their-inspection/
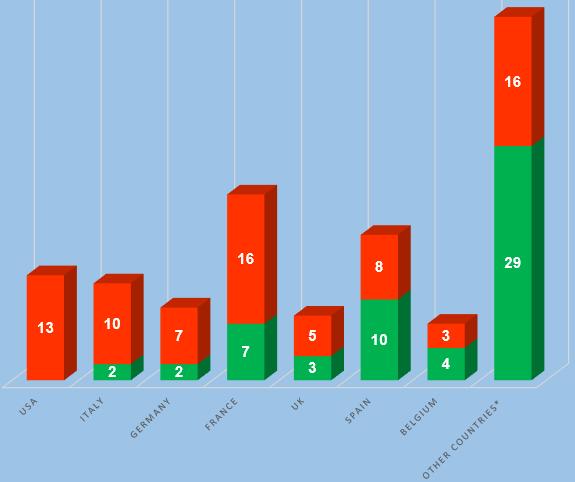
An analysis of the results of inspections of foreign manufacturers in those countries where the largest number of Russian inspections were conducted, now shows the same trends as before. Manufacturing sites located in Spain and Belgium show not bad results; manufacturing sites located in the USA, Italy and Germany show the worst results.
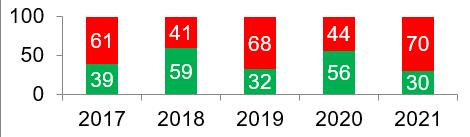
At the same time, according to the currently available data on the results of 30 last year inspections, in 2021 the percentage of refusals to issue GMP-conclusions to foreign manufacturers became the highest in 5 years of inspections. Manufacturers should note that preparing for remote inspection requires additional effort on the part of the site; the successful and timely implementation of the plan of corrective and preventive actions (CAPA plan) depends largely on how soon actions begin to be carried out.
It should be noted the success of Russian manufacturers, who are getting more and more conclusions. According to the register, in the first half of 2022, Russian manufacturers were issued 3 GMP-conclusions [1]. They were obtained by the manufacturing sites of Ruzpharma LLC, Agrobiosnab LLC and GOROS21.RU LLC.
You can read about domestic manufacturers of veterinary medicines that received GMP-conclusions based on the results of inspections performed by the Russian regulator here (RU): https://pharmprom.ru/rossijskie-gmp-sertificirovannye-proizvoditeli-veterinarnyx-preparatov/
In accordance with the inspection schedule published on the FSBI “VGNKI” website 30.06.2022, 36 inspections of manufacturers of veterinary medicinal products are planned for the second half of 2022, which sites located in Australia, Belarus, Bulgaria, Brazil, Hungary, Vietnam, Germany, Israel, Spain, Italy, China, the Netherlands, New Zealand, Serbia, Slovakia, the USA, Finland, France and Czech Republic [2]. Some of these inspections were originally planned for previous years then they were postponed to new dates. 6 inspections rescheduled for the first half of 2023. Most of the inspections should take place in relation to the manufacturing sites located in Spain, China, the USA and France.
Past events and activities
On January 21 this year, by the Resolution of the EEC Council No. 1, the Rules for the regulation of the circulation of veterinary medicinal products in the customs territory of the Eurasian Economic Union were approved [3]. The approved document will enter into force on March 13, 2024. Appendix 26 to it – “Rules for conducting pharmaceutical inspections“. These Rules will establish a common procedure for conducting pharmaceutical inspections of the manufacture of veterinary medicinal products for compliance with the EAEU GMP Rules requirements.
In February, the Resolution of the EEC Council No. 65 of July 14, 2021 “On Amendments to the Rules of Good Manufacturing Practice of the Eurasian Economic Union” came into force, in which Annex 15 to the EAEU GMP Rules “Qualification and Validation” is presented in a new edition [4].
A seminar on changes in approaches to qualification and validation at the enterprise was announced by the FSBI “VGNKI” [5].
What aspects do manufacturers need to pay closer attention to, Alexander Belinsky (currently a leading qualification specialist of PQE Group), recently spoke (RU): https://pharmprom.ru/rossijskie-gmp-sertificirovannye-proizvoditeli-veterinarnyx-preparatov/
In the first half of February, the FSBI “VGNKI” was visited by Jean-Pierre Orand, Director of the French Agency for Veterinary Medicinal Products (Agence Nationale du Médicament Vétérinaire, ANMV) and Marie-Agnès Amos, Deputy Adviser for Agriculture, Assistant for Veterinary, Sanitary, Phytosanitary Issues for Russia, Kazakhstan, Belarus, Armenia, Uzbekistan, Kyrgyzstan of the Economic Service of the French Embassy in Russia. During this visit, Danil Rudniaev, Deputy Director of the FSBI “VGNKI”, explained the procedure for organizing and conducting inspections of manufacturers of medicinal products for compliance with the GMP Rules requirements and answered questions about the reasons for the refusal of French manufacturers of medicinal products for veterinary use to issue a positive conclusion [6].
On the eve of this visit, Jean-Pierre Orand and Marie-Agnès Amos met with Russian representatives of foreign companies whose production sites are located, in particular, in France.

In March, amendments to Federal Law No. 99-FZ of May 04, 2011 “On Licensing Certain Types of Activities” came into force, which provide for a new licensing control procedure for domestic manufacturers of medicinal products “Periodic confirmation of compliance with licensing requirements” [7]. Based on the results of periodic confirmation of compliance, it is possible to obtain a conclusion on compliance with the GMP Rules requirements.
The following month, Decree of the Government of the Russian Federation of April 12, 2022 No. 353 “On the specifics of licensing activities in the Russian Federation in 2022” determined for which licensees the period for periodic (primary) confirmation of compliance with license requirements is extended by 12 months. This decision applies to licensees whose three-year period expires in 2022, after which they must complete the relevant procedure [8, 9].
In April, “Methodological recommendations for preparing for a remote inspection of a foreign manufacturer of medicinal products for veterinary use“ were posted on the FSBI “VGNKI” website. According to these guidelines:
- The applicant and responsible officials of the inspected manufacturing site provide remote interaction using technical means and coordinate them with the head of the commission of inspectors. In doing so, the following should be taken into account:
- using communication platforms to timely provision of data, especially for large files;
- use of videoconferencing for real-time discussion with the manufacturing site personnel;
- the ability to provide video recordings from a camera in real time or video recordings for remote analysis of production operations, equipment, premises and related documentation, indicating the time of video recording;
- the time difference between Moscow and the country in whose territory the inspected manufacturing site is located;
- organization of the work of interpreters (from the official language of the country where the manufacturing site is located into Russian – at least two people) during the entire time of the inspection.
- No later than 5 working days before the start of the inspection of the manufacturing site, the applicant/manufacturer ensures the availability of documents, materials and information in electronic form in Russian, as well as in the original language of the manufacturer (according to the given list) by placing them in the cloud storage with the right of access for review by the commission of inspectors.
- Officials during the inspection period should confirm the geolocation of the inspected manufacturing site, as well as be ready to answer the questions of the inspectors and submit documents at their request in accordance with the plan for conducting the inspection of the manufacture of medicinal products [10].
Additional recommendations on preparing of medicines manufacturers for remote inspection for compliance with the GMP Rules requirements can be found here (RU): https://pharmprom.ru/podgotovka-k-distancionnomu-gmp-inspektirovaniyu/
In mid-April, the anniversary XXX Moscow International Veterinary Congress MVC 2022 was held. As part of this congress, the section “Business and government. Breaking stereotypes” was held [11]. Vladimir Subbotin, Deputy Director of the Department for Sanitary, Phytosanitary and Veterinary Measures of the EEC, spoke in his presentation about the unified rules for regulating the circulation of veterinary medicinal products in the EAEU. He noted that the documents confirming the compliance of the manufacture of veterinary medicinal products with the GMP Rules requirements of the EAEU Member States and issued by authorized bodies in accordance with the legislation of the EAEU Member States before January 1, 2021, are valid until their expiration date, but no later than December 31, 2025 year, and are mutually recognized by the authorized bodies of all Member States.
Semen Zhavoronkov, Executive Director of the Association of Veterinary Pharmaceutical Manufacturers “AVFARM”, in his speech on the topic “What threatens 35% of medicines registered in Russia – manufacturers’ opinion” gave an analysis of the main risks of the new and existing legislation. In particular, he cited the following statistics: if we talk only about GMP inspection of four leading foreign manufacturers of veterinary medicines, then today only 7% of foreign manufacturers hold Russian GMP certificates. If we analyze separately foreign chemical-pharmaceutical manufacturers, only 9% of them have Russian GMP certificates, and 6% of manufacturers of immunobiological veterinary medicines [12].
As of mid-2022, foreign manufacturers have only 29 valid GMP-conclusions, the number of which over the past six months has been approximately at the same level.
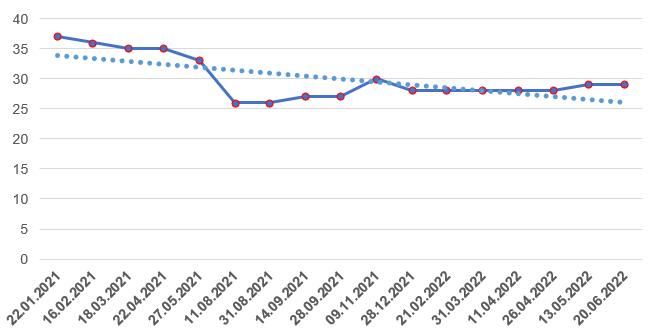
Russian manufacturers currently have 15 valid conclusions.
At the end of April, within the framework of the XI International Pharmaceutical Forum PharmPRO in Moscow, a thematic session “Quality management in the production of medicinal products” was organized. At this session, Irina Krasnova, Deputy Director of the Production Development and Registration Department of NPF Materia Medica Holding LLC, ex- Quality Director, warned the forum participants against entering incomplete or redundant information into the registration dossier [13].
Back in the last year, summing up the four-year activity of the GMP inspectorate at the III All-Russian Pharmprobeg, Danil Rudniaev said that it was the inspection that made it possible to identify the problem – a large number of nonconformities associated with discrepancies in information in the registration dossier and the actual state of affairs on the site; this stimulated pharmaceutical manufacturers to revise their registration dossiers. Today, most manufacturers have done the necessary work, updated their control methods and, in general, began to pay more attention to compliance with quality standards [14].
A round table dedicated to the new rules for the regulation of the circulation of veterinary medicinal products in the customs territory of the Eurasian Economic Union was held at the end of May at the site of All Russia Public Organization “Delovaya Rossiya”. The new rules will come into force in 2024, however, the participants in the veterinary market have accumulated a significant number of questions and comments on the text of the rules. Representatives of the EEC and Rosselkhoznadzor during the round table answered questions from the heads of the Association of Veterinary Pharmaceutical Manufacturers “AVFARM” and the Union of Animal Industry Enterprises (SPZ), as well as the manufacturers of veterinary medicines present. In particular, they discussed approaches to conducting GMP inspections, including in a remote format, the possibility of increasing the deadlines for submitting CAPA plans, as well as the recognition of GMP certificates issued by national authorized bodies. As a result of the round table, the participants agreed to prepare a common resolution for the subsequent development of individual decisions, including work on amending the text of the rules and creating a document with clarifications of certain paragraphs [15].
In June, within the framework of the business program of the exhibition “MVC: Cereals-Mixed Feed-Veterinary-2022”, the conference “State and prospects for solving issues of import substitution in the field of circulation of medicinal products for veterinary use” was held. At this conference, Vasilina Gritsyuk, Deputy Director of the the FSBI “VGNKI”, told how veterinary medicines will be registered under a simplified scheme [16].
In particular, according to Appendix 20 “Peculiarities of licensing regimes in the field of circulation of medicinal products for veterinary use” of Decree of the Government of the Russian Federation of April 12, 2022 No. 353, until September 1, 2023, for medicinal products for veterinary use produced in the Russian Federation for the purpose of import substitution, an accelerated state registration procedure is established, not exceeding 60 working days, due to the refusal to examine the quality of the medicinal product for veterinary use, subject to the submission of a conclusion on compliance of the manufacturing site with the GMP Rules requirements (with the exception of live vaccines and immunobiological products against especially dangerous animal diseases specified in the list of contagious, including especially dangerous, animal diseases for which restrictive measures (quarantine) can be established, approved by the Ministry of Agriculture of the Russian Federation) [9].
Upcoming events and activities
In June, registration was opened for the VII All Russian GMP Conference, which will be held in Irkutsk on September 7-8. The organizer of the conference is the Ministry of Industry and Trade of Russia together with the FSI “SID & GP”. In recent years, the agenda of the conference has included not only Good Manufacturing Practices, but also Good Laboratory, Engineering and other Good Practices (GxP). In addition, round tables and thematic sessions on the veterinary field are held within the framework of the conference. On the second day of work, an extended master class on Good Manufacturing Practices and GMP inspections will be held, which can only be attended in person [17, 18].
On October 21, Moscow will host the IV International Conference “Medicinal products logistics”. In particular, the conference will host the SCM Pharm 2022 Award Ceremony. The SCM Pharm Community of Managers and Specialists in Logistics and Quality is preparing the publication of the GDP Review 3 practical collected articles [19].
On November 9-10, St. Petersburg will host the ISPE EAEU Conference. The main goal of the conference is an open discussion of technical and technological innovations in the field of pharmaceutical production, the latest regulatory trends and practical experience in their implementation. Every year, leading experts of the pharmaceutical industry, representatives of government authorities and professional associations, as well as manufacturing companies from the EAEU and other countries are invited to participate in the business program of the conference [20].
Manufacturers of veterinary medicines are encouraged to take an active part in GM(D)P-related events and activities and prepare more thoroughly for GMP inspections.
The material presented was prepared using data relevant to 30.06.2022. In case of new or additional data the article can be updated.
Konstantin Morozov, GMP Specialist, Auditor of Pharmaceutical Enterprises
REFERENCES:
- Россельхознадзор / Деятельность / Госуслуги / Выдача заключения о соответствии производителя (иностранного производителя) лекарственных средств для ветеринарного применения требованиям правил надлежащей производственной практики / Дополнительная информация для заявителя / Государственный реестр заключений о соответствии производителя (иностранного производителя) лекарственных средств для ветеринарного применения требованиям правил надлежащей производственной практики. URL: https://fsvps.gov.ru/ru/gosuslugi/zaklyuchenie-o-sootvetstvii (дата обращения 30.06.2022)
- ФГБУ «ВГНКИ» / Структура / Отдел инспекции производства на соответствие требованиям надлежащей производственной практики / График проведения инспектирования иностранных производителей на соответствие требованиям надлежащей производственной практики. URL: https://www.vgnki.ru/assets/files/grafik-na-sajt-30062022(1).pdf (дата обращения 30.06.2022)
- Евразийский экономический союз / Решение Совета Евразийской экономической комиссии от 21.01.2022 № 1 «О Правилах регулирования обращения ветеринарных лекарственных средств на таможенной территории Евразийского экономического союза. URL: https://docs.eaeunion.org/Pages/DisplayDocument.aspx?s=%7Be1f13d1d-5914-465c-835f-2aa3762eddda%7D&w=9260b414-defe-45cc-88a3-eb5c73238076&l=%7B8a412e96-924f-4b3c-8321-0d5e767e5f91%7D&EntityID=32004 (дата обращения 30.06.2022)
- Евразийский экономический союз / Решение Совета Евразийской экономической комиссии от 14.07.2021 № 65 «О внесении изменений в Правила надлежащей производственной практики Евразийского экономического союза». URL: https://docs.eaeunion.org/docs/ru-ru/01429875/err_11082021_65 (дата обращения 30.06.2022)
- ФГБУ «ВГНКИ» / Главная / Пресс-центр / Новости / АНОНС: В ФГБУ «ВГНКИ» пройдет семинар, посвященный изменениям в подходах к квалификации и валидации на предприятии. URL: https://vgnki.ru/anons-v-fgbu-vgnki-projdet-seminar-posvyashhennyj-izmeneniyam-v-podhodah-k-kvalifikacii-i-validacii-na-predpriyatii.html (дата обращения 30.06.2022)
- ФГБУ «ВГНКИ» / Главная / Пресс-центр / Новости / ФГБУ «ВГНКИ» посетил директор французского Агентства по надзору за лекарственными средствами для ветеринарного применения (ANMV). URL: https://www.vgnki.ru/fgbu-vgnki-posetil-direktor-francuzskogo-agentstva-po-nadzoru-za-lekarstvennymi-sredstvami-dlya-veterinarnogo-primeneniya-anmv.html (дата обращения 30.06.2022)
- Электронный фонд правовых и нормативно-технических документов / Федеральный закон от 04.05.2011 г. № 99-ФЗ «О лицензировании отдельных видов деятельности» (редакция, действующая с 1 марта 2022 года). URL: https://docs.cntd.ru/document/902276657 (дата обращения 30.06.2022)
- Россельхознадзор / Новости / Россельхознадзор информирует производителей лекарственных средств для ветеринарного применения о продлении срока подтверждения соответствия лицензионным требованиям. URL: https://fsvps.gov.ru/ru/fsvps/news/209623.html (дата обращения 30.06.2022)
- Электронный фонд правовых и нормативно-технических документов / Постановление Правительства РФ от 12.04.2022 г. № 353 «Об особенностях разрешительной деятельности в Российской Федерации в 2022 году» (с изменениями на 15 июня 2022 года). URL: https://docs.cntd.ru/document/728461969 (дата обращения 30.06.2022)
- ФГБУ «ВГНКИ» / Структура / Отдел инспекции производства на соответствие требованиям надлежащей производственной практики / Полезная информация / Методические рекомендации по подготовке к дистанционной инспекции иностранного производителя лекарственных средств для ветеринарного применения. URL: https://www.vgnki.ru/otdel-inspekcii-proizvodstva-na-sootvetstvie-trebovaniyam-nadlezhashhej-proizvodstvennoj-praktiki.html (дата обращения 30.06.2022)
- XXX Московский международный ветеринарный конгресс / Мероприятия / Программа секций / XXX Ветконгресс / Второй день / Бизнес и власть. URL: https://vetcongress.ru/vetcongress30/day2/bus (дата обращения 30.06.2022)
- Зоомедвет / Новости / Что угрожает рынку ветеринарных препаратов в России? URL: https://zoomedvet.ru/?p=7140 (дата обращения 30.06.2022)
- PharmPRO / Блог / Управление качеством фармпрепаратов: итоги тематической сессии PharmPRO. URL: https://pharmpro.pro/blog/kachestvo/upravlenie-kachestvom-farmpreparatov/ (дата обращения обращения 30.06.2022)
- Фармпробег / Новости / Фармпробег-2021 в Суздале поднял тему фармацевтической системы качества. URL: https://pharmprobeg.ru/novosti/farmprobeg-2021-v-suzdale-podnyal-temu-farmatsevticheskoj-sistemy-kachestva/ (дата обращения обращения 30.06.2022)
- Ветеринария и жизнь / Главная / События / Эксперты обсудили новые правила работы единого рынка ветпрепаратов ЕАЭС. URL: https://vetandlife.ru/sobytiya/eksperty-obsudili-novye-pravila-raboty-edinogo-rynka-vetpreparatov-eaes/ (дата обращения 30.06.2022)
- Ветеринария и жизнь / Главная / Зообизнес / Эксперт: как будут регистрировать ветпрепараты по упрощенной схеме. URL: https://vetandlife.ru/sobytiya/ekspert-kak-budut-registrirovat-vetpreparaty-po-uproshhennoj-sheme/ (дата обращения 30.06.2022)
- ФБУ «ГИЛС и НП» / Новости / 2022 / Открыта регистрация на VII Всероссийскую GMP-конференцию. URL: https://gilsinp.ru/?news=otkryta-registratsiya-na-vii-vserossijskuyu-gmp-konferentsiyu (дата обращения 30.06.2022)
- VII Всероссийская GMP-конференция. URL: http://gosgmp.ru/ (дата обращения 30.06.2022)
- SCM Pharm / Проекты / Издания. URL: https://scmpharm.ru/projects/publishing/ (дата обращения 06.2022)
- Конференция ISPE ЕАЭС – 2022. URL: https://conference.ispe.ru/ (дата обращения 30.06.2022)
