
The quarterly review provides information on the inspection of manufacturers of veterinary medicines for compliance with the requirements of the Good Manufacturing Practice (GMP) which is carried out by specialists of the Inspection Body of the Federal State Budgetary Institution “The All-Russian State Center for Quality and Standardization of Veterinary Drugs and Feed” (FSBI “VGNKI”).
Inspection results
Foreign manufacturers
According to the register of conclusions, published on the Rosselkhoznadzor website 21.03.2024 [1], since the beginning of this year, 7 conclusions on compliance with the GMP Rules requirements were issued. These conclusions were made for sites located in located in Argentina, Hungary, China, the Netherlands, Portugal, Romania and the USA.
An analysis of the results of inspections in those countries where the largest number of Russian inspections were carried out shows the following trends:
- the worst results are at the manufacturing sites located in Brazil, the USA, and Germany (more than 80% of refusals to issue a conclusion);
- the best results are at the manufacturing sites located in Israel and Slovenia (no refusal to issue a conclusion).
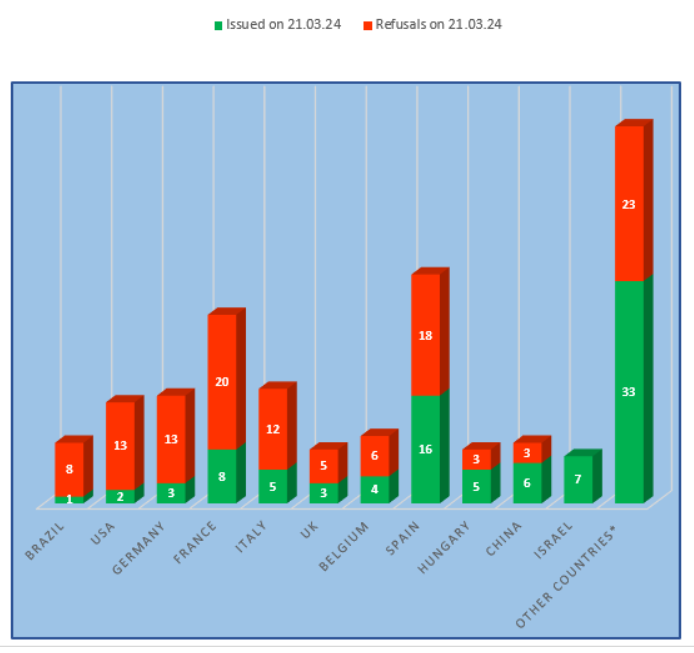
Inspection results for manufacturing sites located in Hungary and China showed some improvement, with refusal rates dropping to 43% and 38% respectively. In the first quarter, sites from Budapest and Qingdao received GMP-conclusions.
The percentage of refusal rate based on the results of inspections carried out at the USA sites also decreased slightly. Perhaps some American sites have begun to more scrupulously study the EAEU GMP Rules, approved by the Resolution of the EEC Council dated 03.11.2016 No. 77 [2] and conduct internal audits for compliance with the requirements of these particular Rules.
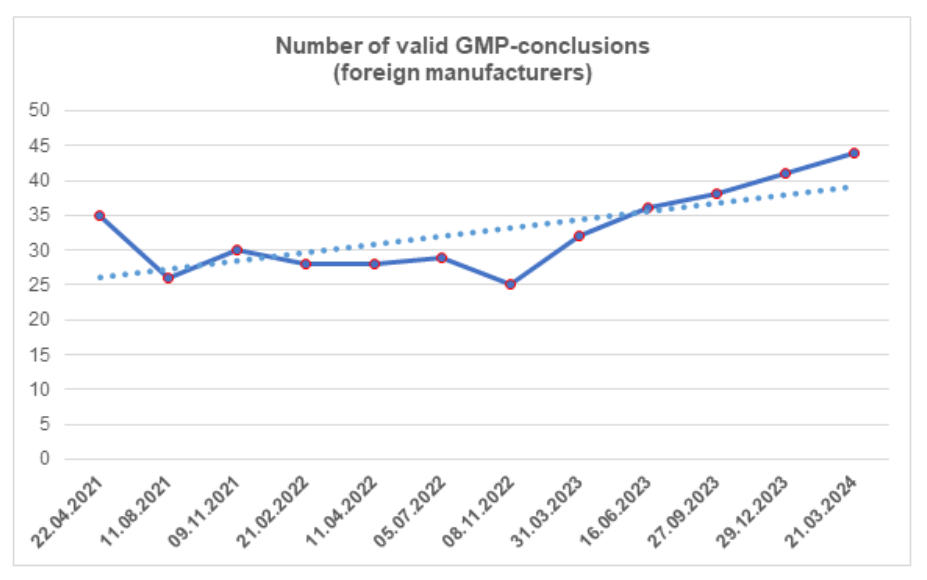
According to the register [1], by the end of the first quarter, the number of valid GMP-conclusions increased. To date, foreign manufacturers have 44 valid GMP-conclusions.
In accordance with the inspection schedule published on the VGNKI website 21.02.2024 [3], 11 inspections of manufacturers of veterinary medicinal products are planned for the second quarter of 2024, which sites located in Hungary, Germany, China, Slovenia, the USA and France.
Russian manufacturers
According to Rosselkhoznadzor [4], currently out of 99 domestic companies, 27 have a GMP certificate. Another 14 manufacturers have already completed the conformity confirmation procedure to further obtain a conclusion.
Past events and activities
In the first quarter of this year, several events and activities took place that might be of interest to manufacturers preparing for inspection.
At the beginning of February, the PharmPRO community of drug manufacturers and suppliers held a free webinar “Quality Management: Current Issues” [5]. The event was devoted to the intricacies of drawing up a registration dossier, the nuances of registering closures, as well as the criteria for conducting an audit of a supplier of packaging materials
In his speech, international expert Aleksandr Aleksandrov dwelt in detail on on-site and remote audits, noting the pros and cons of each of them: “Remote audits are often more effective than in-person audits, because in a remote audit the party being audited does not influence you, it does not influence your assessment” [6].
In February, a group of State Duma deputies from the LDPR introduced a bill for consideration by the chamber to suspend the obligation to obtain a GMP certificate for veterinary medicinal products imported from abroad [7]. According to the draft, it is proposed to suspend the effect of Part 3 of Article 52 of the Federal Law No. 61-FZ of 12.04.2010 “On the Circulation of Medicines” [8], which assumes that the introduction into civil circulation of a medicinal product for veterinary use imported into the Russian Federation is carried out if there is a conclusion on the compliance of the medicinal product manufacturer with the GMP Rules requirements, issued by the authorized federal executive body. Assessing this initiative, the executive director of the AVPHARM association, Semen Zhavoronkov, noted [9]: “As for the industry’s opinion, everyone here understands that today there is no alternative to GMP certification – the whole world certifies manufacturers for compliance with the GMP Rules requirements. In Russia, regulation has also been established for quite a long time, when without a GMP certificate it is impossible to conduct normal activities, that is, to register new veterinary medicines and make changes to the dossiers of already registered ones. In this sense, the introduction of additional verification of whether manufacturers have Russian GMP certificate when introducing medicines into civil circulation from September 1 last year only exacerbated the main problem – the insufficient number of issued certificates.“
“Those enterprises that are interested in maintaining their position in the market of the Russian Federation have done everything necessary to pass the necessary inspections, or are making these efforts to pass it, and there are many such enterprises,” in turn, the executive director of the National Veterinary Association (NVA) Timur Chibilyaev comments on the situation [10].

The State Duma Council recommended submitting the bill to deputies for consideration. The document will be considered at a plenary meeting in May 2024. Until April 9, the State Duma Committee on Health Protection must submit reviews, suggestions and comments to the bill [11].
On March 18, the Federation Council Committee on Agricultural and Food Policy and Environmental Management held a meeting of the working group on legislative support for the domestic production of veterinary medicines, feed and feed additives. According to the First Deputy Chairman of the Federation Council Committee on Agricultural and Food Policy Sergei Mitin, the adoption of this amendment may negatively affect the quality and effectiveness of veterinary medicines, harmlessness to animals and may pose a threat to the biological security of the country. Olga Nikolaicheva, Deputy Director of the Veterinary Department of the Russian Ministry of Agriculture, and Anna Babushkina, Deputy Head of the State Veterinary Supervision Department of Rosselkhoznadzor, gave their assessment of the draft. Following the meeting of the working group, the senators made a collective decision not to support this bill [12].
The federal industry periodical “Veterinary Medicine and Life” described which laws and regulations in the field of veterinary medicine will come into force in March 2024 [13]. In particular, from March 13, the unified “Rules for the regulation of the circulation of veterinary medicinal products in the customs territory of the EAEU”, approved by the Resolution of the EEC Council dated 21.01.2022 No. 1 [14], will come into effect for the EAEU countries.
The AVPHARM Association presented a concise summary of changes, including in the field of GMP certification [15]: “In each EAEU country, national inspection bodies will be created that will conduct independent (for local manufacturers) and joint (for manufacturers outside the EAEU) inspections and confirm compliance of enterprises with the EAEU GMP Rules requirements [2]. Specialists with the necessary qualifications will be combined into the general register of EAEU inspectors.”
In February, an inspectorate training center began operating on the basis of the Eurasian Academy of Good Practices. The first training event of the new center was the intensive “Validation and qualification in pharmaceutical production.” The inspectorate training center, created on the basis of the Academy, became the first specialized GMP center of its kind for the pharmaceutical inspectorate in Russia and throughout the EAEU [16].
In March, on the platform of the Academy, the next advanced training course “Training auditors of the production of medicinal products” was held (correspondence form of education using distance learning technologies) [17].
In the first quarter, free video-meetings “GMP/GDP Q&A” were again held with Aleksandr Aleksandrov. For example, in February, the topic “Documents and records. Accounting, handling and ensuring data integrity” was discussed.
Upcoming events and activities
On April 16-18, Moscow will host the 22nd International Exhibition “Analytics Expo 2024” – the only exhibition in Russia of laboratory equipment and chemical reagents for production and research laboratories in various industries [18].
On April 17, as part of the exhibition, a “Forum for specialists in quality control of medicinal products” will be organized, the partner of which will be the GxP Training Center. The forum is planned to discuss the following topics: “Review of storage requirements for finished products“, “A comprehensive look at pharmaceutical substances: current legal issues and the formation of a registration dossier“, “Methods for quality control of medicinal products for bacterial endotoxins and pyrogenic substances. New methods, directions and reagents of recent years.“
On April 18, Moscow will host the XIII International Pharmaceutical Forum PharmPRO 2024. This is an industry platform for communication and exchange of experience between manufacturers and suppliers [19].
Several special sessions will be organized within the framework of the forum, including the session “Consistent quality of pharmaceuticals at every stage.” At this session, it is planned to consider such topics as “Risk management when replacing the manufacturer of raw materials and materials with alternative ones”, “Digitalization of validation processes”, “Registration aspects within the framework of GMP inspections”, etc.
Manufacturers are encouraged to take an active part in GM(D)P-related events and activities and prepare more thoroughly for GMP inspections.
The material presented was prepared using data relevant to 24.03.2024. In case of new or additional data the article can be updated.
The Federal State Budgetary Institution “The All-Russian State Center for Quality and Standardization of Veterinary Drugs and Feed”, FSBI “VGNKI” is an institution authorized to conduct inspections of manufacturers of medicinal products for veterinary use manufactured outside the Russian Federation for compliance with the GMP Rules requirements
The National Veterinary Association, NVA is a non-profit organization that is a collaboration of key biopharmaceutical companies
The Association of Veterinary Pharmaceutical Manufacturers AVPHARM is an association representing the interests of leading international pharmaceutical companies in the Russian Federation – manufacturers of medicinal products for veterinary use (MSD Animal Health, Zoetis and Boehringer Ingelheim)
REFERENCES:
- Россельхознадзор / Деятельность / Госуслуги / Выдача заключения о соответствии производителя (иностранного производителя) лекарственных средств для ветеринарного применения требованиям правил надлежащей производственной практики / Дополнительная информация для заявителя / Государственный реестр заключений о соответствии производителя (иностранного производителя) лекарственных средств для ветеринарного применения требованиям правил надлежащей производственной практики. URL: https://fsvps.gov.ru/ru/gosuslugi/zaklyuchenie-o-sootvetstvii (дата обращения 24.03.2024)
- Евразийский экономический союз / Решение Совета Евразийской экономической комиссии от 03.11.2016 г. № 77 «Об утверждении Правил надлежащей производственной практики Евразийского экономического союза». URL: https://docs.eaeunion.org/docs/ru-ru/01411921/cncd_21112016_77 (дата обращения 24.03.2024)
- ФГБУ «ВГНКИ» / Структура / Отдел инспекции производства на соответствие требованиям надлежащей производственной практики / График проведения инспектирования иностранных производителей на соответствие требованиям надлежащей производственной практики. URL: https://www.vgnki.ru/assets/2024/files/grafik–na–sajt-21022024.pdf (дата обращения 24.03.2024)
- Россельхознадзор / Россельхознадзор фиксирует почти трехкратный рост количества зарегистрированных отечественных ветеринарных препаратов. URL: https://fsvps.gov.ru/news/rosselhoznadzor–fiksiruet–pochti–trehkratnyj–rost–kolichestva–zaregistrirovannyh–otechestvennyh–veterinarnyh–preparatov/ (дата обращения 24.03.2024)
- PharmPRO / Вебинары / Управление качеством: актуальные вопросы. URL: https://events.pharmpro.pro/vebinar–quality-020224 (дата обращения 24.03.2024)
- Фармпром / Экспертный материал / «Серые» зоны в управлении качеством на фармпроизводстве. URL: https://pharmprom.ru/serye–zony–v–upravlenii–kachestvom–na–farmacevticheskom–proizvodstve/ (дата обращения 24.03.2024)
- ТАСС / ЛДПР предложила отменить обязательный сертификат соответствия для зарубежных ветлекарств. URL: https://tass.ru/obschestvo/20025125 (дата обращения 24.03.2024)
- Электронный фонд правовых и нормативно-технических документов / Федеральный закон от 12.04.2010 г. № 61-ФЗ «Об обращении лекарственных средств» (с изменениями на 30 января 2024 года). URL: https://docs.cntd.ru/document/902209774 (дата обращения 24.03.2024)
- Milknews / Мнения / Семен Жаворонков, АВФАРМ На сегодняшний день альтернативы GMP-сертификации не существует. URL: https://milknews.ru/interviu-i-blogi/zhavoronkov-minenie.html (дата обращения 24.03.2024)
- Сектор Медиа / Новости / Сельское хозяйство / Эксперты / Сельское хозяйство / «Продажа импортных препаратов без сертификата GMP навредит российским производителям». URL: https://sectormedia.ru/news/eksperty–selskoe–khozyaystvo/prodazha–importnykh–preparatov–bez–sertifikata–gmp–navredit–rossiyskim–proizvoditelyam/ (дата обращения 24.03.2024)
- Фармацевтический вестник / Новости / Регуляторика / Госдума рассмотрит в мае проект о продаже ветпрепаратов без специальной сертификации. URL: https://pharmvestnik.ru/content/news/Gosduma–rassmotrit–v–mae–proekt–o–prodaje–vetpreparatov–bez–specialnoi–sertifikacii.html (дата обращения 24.03.2024)
- Фармпром / Регуляторы фармрынка / Сенаторы не поддержали законопроект об отмене сертификата GMP для импортных ветпрепаратов. URL: https://pharmprom.ru/senatory–ne–podderzhali–zakonoproekt–ob–otmene–sertifikata–gmp–dlya–importnyx–vetpreparatov/ (дата обращения 24.03.2024)
- Ветеринария и жизнь / Законодательство / Какие законы и правила в сфере ветеринарии вступают в силу с 1 марта 2024 года. URL: https://vetandlife.ru/sobytiya/kakie–zakony–i–pravila–v–sfere–veterinarii–vstupajut–v–silu–s-1-marta-2024-goda/ (дата обращения 24.03.2024)
- Евразийский экономический союз / Решение Совета Евразийской экономической комиссии от 21.01.2022 № 1 «О Правилах регулирования обращения ветеринарных лекарственных средств на таможенной территории Евразийского экономического союза. URL: https://docs.eaeunion.org/Pages/DisplayDocument.aspx?s=%7Be1f13d1d-5914-465c-835f-2aa3762eddda%7D&w=9260b414-defe-45cc-88a3-eb5c73238076&l=%7B8a412e96-924f-4b3c-8321-0d5e767e5f91%7D&EntityID=32004 (дата обращения 24.03.2024)
- Зооинформ / Нвости / С 13 марта вступают в силу новые правила регулирования рынка ветпрепаратов для ЕАЭС. URL: https://zooinform.ru/v–avfarm–obyasnili–kak–s-13-marta–izmenitsya–regulirovanie–rynka–vetpreparatov/ (дата обращения 24.03.2024)
- Евразийская Академия надлежащих практик / Новости / На базе Евразийской Академии надлежащих практик начал работу центр по обучению инспектората. URL: https://gxp–academy.org/news/na–baze–evraziyskoy–akademii–nadlezhashchikh–praktik–nachal–rabotu–tsentr–po–obucheniyu–inspektorata/ (дата обращения 24.03.2024)
- Евразийская Академия надлежащих практик / Образовательная деятельность / Курсы ПК / «Подготовка аудиторов производства лекарственных средств». URL: https://gxp–academy.org/education/courses/podgotovka–auditorov–proizvodstva–lekarstvennykh–sredstv-/ (дата обращения 24.03.2024)
- Аналитика Экспо / Деловая программа / Форум для специалистов по контролю качества лекарственных средств. URL: https://analitikaexpo.com/ru/agenda/bp_24/sessions24/Lecture2.2/ (дата обращения 24.03.2024)
- PharmPRO / XIII фармацевтический форум PharmPRO. URL: https://events.pharmpro.pro/forum-2024?roistat_visit=395104 (дата обращения 24.03.2024)
