
The review provides available information on the inspection of manufacturers of veterinary medicines for compliance with the requirements of the Good Manufacturing Practice (GMP) which is carried out by specialists of the Inspection Body of the Federal State Budgetary Institution “The All-Russian State Center for Quality and Standardization of Veterinary Drugs and Feed” (FSBI “VGNKI”).
Inspection results
Foreign manufacturers
In 2025, specialists from the FSBI “VGNKI” carried out 29 on-site inspections of foreign manufacturers of veterinary medicines in Austria, Argentina, Brazil, Hungary, Vietnam, Israel, India, Spain, Italy, China, the Netherlands, New Zealand, Uruguay, France, and Switzerland [1].
The last update to the state register of conclusions of compliance of veterinary medicinal product manufacturers with the GMP Rules requirements on the Rosselkhoznadzor website was 25.07.2025 [2]. Further information can be found in the Galen system [3]. Today foreign manufacturers have more than 50 valid GMP-conclusions.
Approximately a quarter of valid GMP-conclusions are held by sites located in countries friendly to Russia (including Argentina, Belarus, Brazil, India and China). At the same time, the number of veterinary medicines from friendly countries registered in Russia has increased by 2.1 times over the past three years: from 139 in 2023 to 295 in 2025. Timur Chibilyaev, Executive director of the National Veterinary Association (NVA) spoke about this in November [4]. The number of veterinary medicines with valid marketing authorizations from unfriendly countries has decreased by 1.2 times (from 750 to 579). The number of suppliers from these regions has decreased by 3.7 times.
The largest number of valid GMP-conclusions belong to manufacturing sites located in China, Spain, and the United States. Approximately 30% of all valid conclusions belong to sites and CMOs of the American companies Elanco and Zoetis (the latter is a member of the AVPHARM association) in various countries. The executive director of the AVPHARM association, Semen Zhavoronkov, noted: “More than 30% of the total number of GMP-inspections are conducted at the sites of three members of our association.”

In accordance with the inspection schedule published on the VGNKI website 16.12.2025 [5], 4 inspections of manufacturers of veterinary medicinal products are planned for the first quarter of 2026, which sites located in in China, Serbia, Slovakia and France.
Russian manufacturers
Since the beginning of the year, 103 veterinary medicines have been registered in Russia, Deputy Director of the FSBI “VGNKI” Vasilina Gritsyuk reported in November. [6]. Over the past three years, the number of veterinary medicines introduced to the market has increased 2.1-fold: from 69 in 2022 to 147 in 2024. This pace was facilitated by the simplification of the registration procedure for domestic medicines for animals. The Decree of the Government of the Russian Federation dated 12.03.2022 No. 353 “On the Specifics of Licensing Activities in the Russian Federation” [7] establishes for domestic veterinary medicines produced for the purpose of import substitution an accelerated state registration procedure, which does not exceed 60 working days and is valid until December 31, 2025. Russian manufacturers can register all medicinal products under an accelerated procedure, except for live vaccines and immunobiological products against particularly dangerous animal diseases. However, the enterprise must have a GMP-conclusion.
Today domestic manufacturers have more than 20 valid GMP-conclusions.
According to the analytical company RNC Pharma [8], the share of domestic manufacturers in the Russian retail veterinary medicines market (including online) continued to increase in the first three quarters of 2025. During the reporting period, they accounted for approximately 55% of the monetary value, while their share in physical units exceeded 74%. Furthermore, there are 17 companies in Russia producing medicines for both animals and humans.
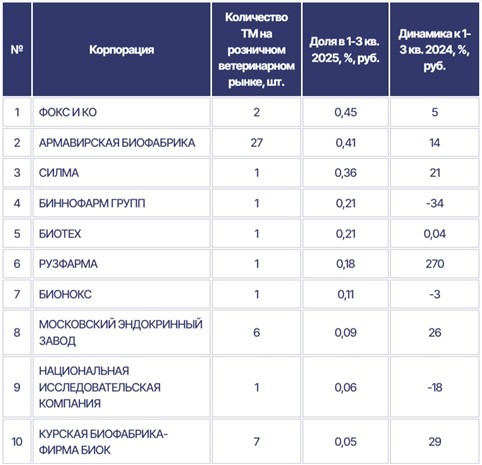
The share of Russian veterinary vaccines on the domestic market has grown to 72.6%, Sergei Dankvert, head of Rosselkhoznadzor, announced during an open dialogue in the Federation Council [9]. “The mere fact that vaccines are of foreign origin does not guarantee their effectiveness,” he noted. “For example, not long ago, large shipments of poultry vaccines from Israel had to be stopped because the manufacturer failed the inspection for compliance with the GMP Rules requirements.”
Past events and activities
In November-December of this year, several events and activities took place that might be of interest to manufacturers preparing for inspection.
In early November, it was announced that the Russian Government had adopted the Decree dated 29.10.2025 No. 1681 “On Establishing the Fee for Issuing Certificates (Conclusions) of Conformity of the Production of Medicinal Products for Veterinary Use with the Requirements of the Good Manufacturing Practice Rules of the Eurasian Economic Union” [10]. The fee for issuing EAEU GMP-certificates to manufacturers of veterinary medicines is 23,300 rubles. The resolution will enter into force on March 1, 2026.
In November, the Russian Government adopted the Decree dated 08.11.2025 No. 1766 “On the Repeal of Certain Acts of the Government of the Russian Federation” [11]. In particular, in accordance with the adopted Decree, Government Decree dated 03.12.2015 No. 1314 “On Determining the Compliance of Manufacturers of Medicinal Products with the Requirements of the Good Manufacturing Practice Rules” [12] shall ceases to be in effect as of March 1, 2026. It should be noted [13] that from March 1, 2026, pharmaceutical inspections should begin to be carried out in accordance with the EAEU Rules with the issuance of GMP-certificates (Appendix No. 26 to the Decision of the EEC Council dated 21.01.2022 No. 1 “On the Rules for Regulating the Circulation of Veterinary Medicinal Products in the Customs Territory of the Eurasian Economic Union”) [14].
That same month, the Eurasian Academy of Good Practices held a free webinar, “Implementation of AI in the Pharmaceutical Industry in the Context of GMP” [15]. The webinar’s presenters, Konstantin Koshechkin and Ruslan Kalashnikov, focused on specific, practical cases of applying artificial intelligence (AI) in the context of strict GMP regulation.

Let us recall that in July of this year, a draft of Annex 22 to the EU GMP “Artificial Intelligence” was published [16, 17]. In light of the rapid advancement of digital technologies and the implementation of AI systems in pharmaceutical manufacturing, the update of GMP guidelines is essential to ensure that they continue to provide clear, practical and relevant guidance for manufacturers and national competent authorities. The new Annex on Artificial Intelligence establishes requirements for the use of AI and machine learning in the manufacturing of active substances and medicinal products. It sets up requirements for the selection, training, and validation of AI models. Emphasis is made on the definition of the intended use of the model, the establishment of performance metrics, the quality of model training data, and the management and processing of test data. Annex 22 foresees a continuous oversight of AI systems, including change control, model performance monitoring and procedures for human review when necessary [18].
At the end of November, Moscow hosted the international exhibition of equipment, raw materials, and technologies for pharmaceutical production, Pharmtech & Ingredients 2025. Several useful events took place on the first day of the exhibition [19]. Speaking at the “ColdChain: Pharmaceutical Logistics” discussion session, Tadzio Schilling, CEO of the Association of European Businesses (AEB), discussed the challenges and prospects of human use and veterinary medicines logistics. He noted that both in Russia and Western countries, the pharmaceutical industry, and healthcare in general, is perceived as a particularly important sector, which is why, despite external challenges, foreign companies continue to operate in Russia. Anna Filippova, Director of Pharmaceutical Sector Development at WELLGO, discussed the specifics of pharmaceutical logistics using different modes of transport. Andrey Kukharenko, Director of Development at Cold Chain Technologies, discussed the use of Artificial Intelligence in cold chain logistics for medicinal products.

You can read more about the cold chain of medicinal products here:
The exhibition’s business program also included a roundtable discussion on the veterinary medicines market, titled “From Import Substitution to Technological Leadership: New Challenges for Russian Veterinary Pharmaceuticals.” Timur Chibilyaev presented a report on veterinary medicines production, emphasizing the growing interest of the human use sector in the veterinary industry. Semen Zhavoronkov delivered a report, “Veterinary Medicines from International Manufacturers in the EAEU: New Challenges and Prospects.” The expert noted that foreign exports to Russia continue to recover after tightened access conditions. He concluded that business is currently impacted by:
- aligning registration dossiers;
- preparing for joint GMP-inspections.
In December, the GхP Training Center held the “Validation Specialists Forum” in Moscow. The event brought together specialists from various sectors of the pharmaceutical industry involved in validation processes. The forum provided a unique platform for exchanging experiences, discussing current issues and trends in validation, and exploring new ideas and solutions.
Challenges in 2026
As noted above, preparation for joint GMP inspections within the EAEU is one of the main challenges for next year. Some questions from veterinary medicines manufacturers were already raised at the X All-Russian GMP Conference [20] during the panel session “EAEU’s common market of medicinal products for veterinary use” [21]:
- Practical implementation of the joint inspection mechanism – when will the first inspections take place under Appendix No. 26 to the Decision of the EEC Council dated 21.01.2022 No. 1 [14]?
- How will the mechanism for concluding an agreement(s) with inspectorates be implemented: calculation of the cost of inspection, the procedure for concluding agreements?
- Is it possible to extend the time allotted for preparing the CAPA plan?
- Timeframe for the inspection: is it possible to set a deadline for the joint inspection from the moment the decision is made?
Experts’ proposals for Appendix No. 26 to the Decision of the EEC Council dated 21.01.2022 No. 1 were presented this year during a working meeting at the Russian Union of Industrialists and Entrepreneurs (RSPP) [22].
This is the final review of Russian GMP-inspections of veterinary medicines manufacturers. Manufacturers are encouraged to take an active part in GM(D)P-related events and activities and prepare more thoroughly for GMP-inspections. We wish them patience and good luck in the New Year!

The Federal State Budgetary Institution “The All-Russian State Center for Quality and Standardization of Veterinary Drugs and Feed”, FSBI “VGNKI” is an institution authorized to conduct inspections of manufacturers of medicinal products for veterinary use manufactured outside the Russian Federation for compliance with the GMP Rules requirements
The National Veterinary Association, NVA is a non-profit organization that is a collaboration of key biopharmaceutical companies
The Association of Veterinary Pharmaceutical Manufacturers, AVPHARM is an association representing the interests of leading international pharmaceutical companies in the Russian Federation – manufacturers of medicinal products for veterinary use (MSD Animal Health, Zoetis and Boehringer Ingelheim)
The Association of European Businesses (AEB) is an independent non-profit organization uniting more than 380 companies operating in Russia and the main representative office of foreign investors in Russia
References:
- ФГБУ «ВГНКИ» / Пресс-центр / Новости / ФГБУ «ВГНКИ» провело 29 инспекций иностранных производителей ветпрепаратов в текущем году. URL: https://www.vgnki.ru/fgbu-vgnki-provelo-29-inspekcij-inostrannyh-proizvoditelej-vetpreparatov-v-tekushhem-godu.html (Accessed on 20.12.2025)
- Россельхознадзор / Деятельность / Госуслуги / Выдача заключения о соответствии производителя (иностранного производителя) лекарственных средств для ветеринарного применения требованиям правил надлежащей производственной практики / Дополнительная информация для заявителя / Государственный реестр заключений о соответствии производителя (иностранного производителя) лекарственных средств для ветеринарного применения требованиям правил надлежащей производственной практики. URL: https://fsvps.gov.ru/files/gosudarstvennyj-reestr-zakljuchenij-o-sootvetstvii-proizvoditelja-inostrannogo-proizvoditelja-lekarstvennyh-sredstv-dlja-veterinarnogo-primenenija-trebovanijam-pravil-nadlezhashhej-proizvodstvennoj-pr/ (Accessed on 20.12.2025)
- Автоматизированная система Гален / Заключения GMP / Государственный реестр заключений о соответствии производителя (иностранного производителя) лекарственных средств для ветеринарного применения требованиям правил надлежащей производственной практики. URL: https://galen.vetrf.ru/react/registry/gmp/registry (Accessed on 20.12.2025)
- Ветеринария и жизнь / Наука и технологии / Ветпрепаратов из дружественных стран за три года стало больше в два раза. URL: https://vetandlife.ru/pet-business/vetpreparatov-iz-druzhestvennyh-stran-za-tri-goda-stalo-bolshe-v-dva-raza/ (Accessed on 20.12.2025)
- ФГБУ «ВГНКИ» / Структура / Отдел инспекции производства на соответствие требованиям надлежащей производственной практики / График проведения инспектирования иностранных производителей на соответствие требованиям надлежащей производственной практики. URL: https://www.vgnki.ru/assets/2025/files/grafik-na-sajt-16122025.pdf (Accessed on 20.12.2025)
- Ветеринария и жизнь / Зообизнес / Сколько новых лекарств для животных зарегистрировали в РФ в 2025 году. URL: https://vetandlife.ru/pet-business/skolko-novyh-lekarstv-dlya-zhivotnyh-zaregistrirovali-v-rf-v-2025-godu/ (Accessed on 20.12.2025)
- КонсультантПлюс / Постановление Правительства РФ от 12.03.2022 N 353 (ред. от 02.10.2025) “Об особенностях разрешительной деятельности в Российской Федерации”. URL: https://www.consultant.ru/document/cons_doc_LAW_411447/ (Accessed on 20.12.2025)
- RNC Pharma / Российские фармпроизводители обеспечивают порядка 2,2% от денежного объема ветеринарной розницы и сильно отстают от профильных игроков с точки зрения динамики продаж. URL: https://rncph.ru/blog/191125/ (Accessed on 20.12.2025)
- Ветеринария и жизнь / Новости / Доля отечественных ветеринарных вакцин достигла 72,6% – Россельхознадзор. URL: https://vetandlife.ru/livestock/dolya-otechestvennyh-veterinarnyh-vakcin-dostigla-72-6-rosselhoznadzor/ (Accessed on 20.12.2025)
- КонсультантПлюс / Постановление Правительства РФ от 29.10.2025 N 1681 «Об установлении размера платы за выдачу сертификатов (заключений) соответствия производства лекарственных средств для ветеринарного применения требованиям правил надлежащей производственной практики Евразийского экономического союза». URL: https://www.consultant.ru/document/cons_doc_LAW_518017/ (Accessed on 20.12.2025)
- КонсультантПлюс / Постановление Правительства РФ от 08.11.2025 N 1766 «О признании утратившими силу некоторых актов Правительства Российской Федерации». URL: https://www.consultant.ru/document/cons_doc_LAW_518691/ (Accessed on 20.12.2025)
- КонсультантПлюс / Постановление Правительства РФ от 03.12.2015 N 1314 (ред. от 05.09.2020) «Об определении соответствия производителей лекарственных средств требованиям правил надлежащей производственной практики» (вместе с «Правилами организации и проведения инспектирования производителей лекарственных средств на соответствие требованиям правил надлежащей производственной практики, а также выдачи заключений о соответствии производителя лекарственных средств указанным требованиям»). URL: https://www.consultant.ru/document/cons_doc_LAW_190256/ (Accessed on 20.12.2025)
- ФармПром.РФ / Регуляторы фармрынка / Изменения в правилах GMP-инспектирования производителей лекарственных средств. URL: https://pharmprom.news/izmeneniya-v-pravilax-gmp-inspektirovaniya-proizvoditelej-lekarstvennyx-sredstv/ (Accessed on 20.12.2025)
- Евразийский экономический союз / Решение Совета Евразийской экономической комиссии от 21.01.2022 № 1 «О Правилах регулирования обращения ветеринарных лекарственных средств на таможенной территории Евразийского экономического союза. URL: https://docs.eaeunion.org/Pages/DisplayDocument.aspx?s=%7Be1f13d1d-5914-465c-835f-2aa3762eddda%7D&w=9260b414-defe-45cc-88a3-eb5c73238076&l=%7B8a412e96-924f-4b3c-8321-0d5e767e5f91%7D&EntityID=32004 (Accessed on 20.12.2025)
- Евразийская Академия надлежащих практик / Образовательная деятельность / Вебинары / Внедрение ИИ в фармацевтической индустрии в контексте GMP. URL: https://gxp-academy.org/education/webinars/vnedrenie-ii-v-farmatsevticheskoy-industrii-v-kontekste-gmp/ (Accessed on 20.12.2025)
- ФармПром.РФ / Фарминдустрия в мире / Искуственный интеллект в европейских правилах GMP – Обзор проекта Приложения 22. URL: https://pharmprom.news/iskustvennyj-intellekt-v-evropejskix-pravilax-gmp-obzor-proekta-prilozheniya-22/ (Accessed on 20.12.2025)
- EC / Draft guidelines: New annex 22 – Artificial intelligence. URL: https://health.ec.europa.eu/document/download/5f38a92d-bb8e-4264-8898-ea076e926db6_en?filename=mp_vol4_chap4_annex22_consultation_guideline_en.pdf (Accessed on 20.12.2025)
- EC / Stakeholders’ Consultation on EudraLex Volume 4 – Good Manufacturing Practice Guidelines: Chapter 4, Annex 11 and New Annex 22. URL: https://health.ec.europa.eu/consultations/stakeholders-consultation-eudralex-volume-4-good-manufacturing-practice-guidelines-chapter-4-annex_en (Accessed on 20.12.2025)
- Pharmtech & Ingredients / Итоги первого дня работы выставки Pharmtech & Ingredients 2025. URL: https://pharmtech-expo.ru/ru/media/news/2025/november/25/pharmtech-2025-itogi-pervogo-dnya/ (Accessed on 20.12.2025)
- X Всероссийская GMP-конференция. URL: http://gosgmp.ru/ (Accessed on 20.12.2025)
- PharmProm Net / Events / Session “EAEU’s common market of medicinal products for veterinary use” at the All-Russian GMP Conference 2025. URL: https://pharmprom.net/session-eaeus-common-market-of-medicinal-products-for-veterinary-use-at-the-all-russian-gmp-conference-2025/ (Accessed on 20.12.2025)
- PharmProm Net / Regulatory News / Experts propose changes to the rules for regulating veterinary medicinal products in the EAEU. URL: https://pharmprom.net/experts-propose-changes-to-the-rules-for-regulating-veterinary-medicinal-products-in-the-eaeu/ (Accessed on 20.12.2025)

