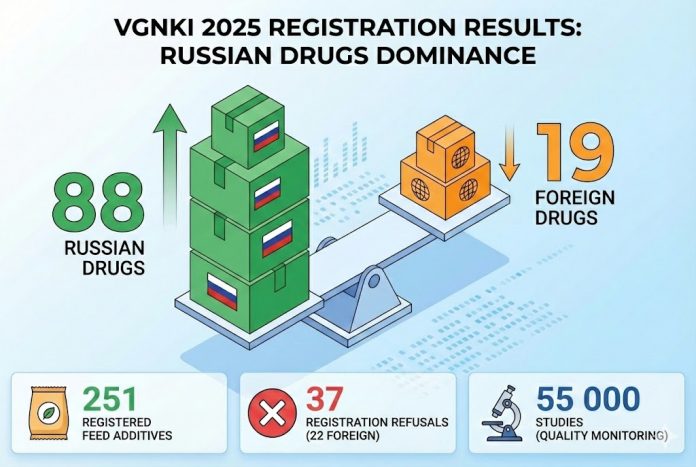
The VGNKI (Russian State Center for Quality and Standardization of Veterinary Drugs and Feed) presented statistics for 2025, confirming a dramatic market shift. The number of new Russian drugs exceeded foreign analogs by 4.5 times, while controls on import safety were significantly tightened.
Key Registration Indicators 2025
The institution, subordinate to Rosselkhoznadzor, continues to filter the market, allowing only proven effective remedies into circulation. Below is the summary statistics of the agency’s activities for the reporting period.
| Category / Indicator | 2025 Value | Comment |
|---|---|---|
| Russian Veterinary Drugs | 88 | Growth in local manufacturer share |
| Foreign Veterinary Drugs | 19 | Decline in importer activity |
| Feed Additives | 251 | Based on the analysis of 288 dossiers |
| Registration Refusals | 37 | 22 were foreign drugs (unconfirmed safety) |
Quality Control and Laboratory Monitoring
As part of ensuring biological safety, VGNKI conducted extensive work on verifying products in circulation. The Testing Center performed over 55,000 studies.
“The share of detected non-compliances within the state assignment was 3%. However, for commercial orders, this figure reached 10.3%, indicating high risks in the unregulated sector. The majority of violations are found in feed and feed additives,” the agency’s report notes.
R&D and Scientific Achievements
In addition to control and supervisory activities, the institution is actively developing its scientific direction:
- Aquaculture Vaccine: Jointly with ARRIAH, the “ARRIAH-AQUARUS-6” vaccine for salmon protection was registered.
- Industrial Release: Three batches of colibacteriosis vaccine were introduced into civil circulation.
- Intellectual Property: 3 patents for inventions were obtained, and over 200 scientific papers were published.
Source: Veterinary and Life