This year, specialists of the Federal State Budgetary Institution “The All-Russian State Center for Quality and Standardization of Veterinary Drugs and Feed” (FSBI “VGNKI”) subordinated to Rosselkhoznadzor, conducted more than 40 inspections of veterinary manufacturers for compliance with the requirements of the Good Manufacturing Practice (GMP). This review provides information on inspection, as well as some tips and tricks that may be useful to manufacturers in preparing for inspections.
Inspection results
Foreign manufacturers
According to the register of issued conclusions, published on the Rosselkhoznadzor website 27.12.2022 [1], in the second half of this year, 6 conclusions on compliance with the GMP Rules requirements were issued. These conclusions were made for sites located in Brazil, China, Italy and France. In total, 9 GMP conclusions were issued in 2022.
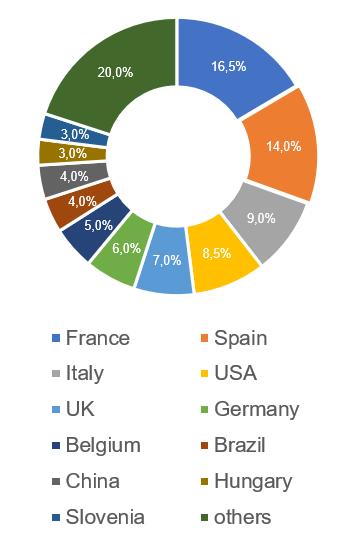
The largest number of Russian inspections, based on the results of which, in 2017-2022, decisions were made to issue/refuse to issue a GMP-conclusion (25, which is 16,5%), were carried out at manufacturing sites located in France. To date, the French sites also have the largest number of valid conclusions (6).
For the record:
The quality of medicinal products for veterinary use distributed in France is monitored by the National Agency for Veterinary Medicinal Products (ANMV). Thanks to its expertise, ANMV is an Agency highly involved in European as well as international activities. Thus, in 2019, at a panel discussion within the framework of the IV All-Russian GMP Conference, Inspector of manufacturers of veterinary medicines Vincent Neuviale presented information on GMP inspection conducted by ANMV in France. At the beginning of 2022, the director of the Agency paid a working visit to FSBI “VGNKI”. ANMV pays great attention to the results of inspections carried out by the Russian inspectorate at French manufacturing sites.
You can also read about French manufacturers of veterinary medicines and the ANMV agency here.
An analysis of the results of inspections in those countries where the largest number of Russian inspections were carried out now shows the following trends:
– The worst results are at the manufacturing sites located in the USA (100% of refusals to issue a conclusion), Brazil and Germany (more than 80% of refusals to issue a conclusion).
– The best results are at the manufacturing sites located in Slovenia (no refusal to issue a conclusion), as well as in Hungary and China (no more than 33% of refusals to issue a conclusion).
At the same time, the results of a number of inspections conducted in the second half of the year may become known only in the first half of 2023.
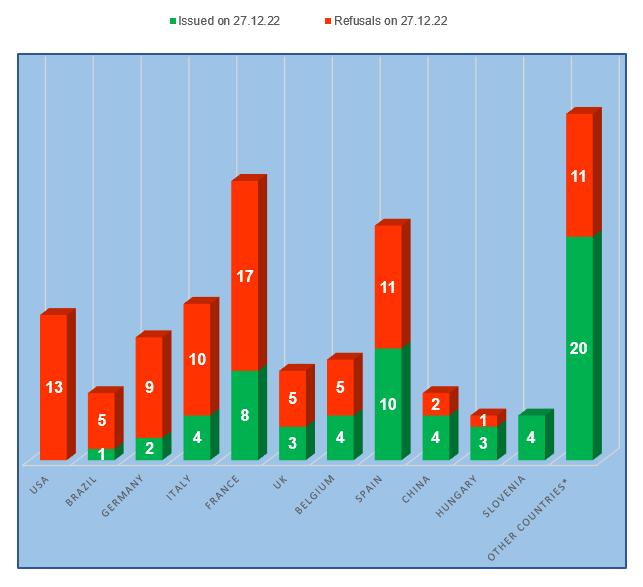
Perhaps manufacturers from Eastern European countries understand Russian requirements better. In addition, it may be easier for a manufacturer who is fully localized in the Russian Federation to prepare for and pass Russian inspections. The success of KRKA company from Slovenia should be especially noted. The history of this company in Russia goes back several decades. To date, the plant of KRKA-RUS LLC in Istra is one of the most modern plants in Russia; according to data for 2021, the total investment in the development and construction of this plant amounted to more than 200 million euros [2]. According to the analytical company RNC Pharma [3], from January to October 2022, KRKA was the absolute leader in terms of natural dynamics of deliveries of veterinary medicines to Russia (in packages), while the company showed one of the most notable indicators in terms of shipment dynamics (growth 2.3 times). Interestingly, the first Russian GMP inspection of foreign manufacturers of medicinal products for human use took place at the KRKA plant in Slovenia. Not surprisingly, quality preparation for Russian inspections is a high priority for this company.
In Hungary, the results of Russian inspections of manufacturers of medicinal products for veterinary use are closely monitored by the Directorate of Veterinary Medicinal Products, a division of the National Food Chain Safety Office (NÉBIH), which is in close contact with manufacturers.
As for manufacturers from China, Chinese companies may be motivated to prepare well for Russian inspections if they want to replace the supply of products from countries unfriendly to Russia. Back in March of this year, when discussing the supply of veterinary medicines with representatives of the All-Russian Non-Governmental Organization of Small and Medium Business “Opora Russia”, the Head of Rosselkhoznadzor Sergey Dankvert noted that today Chinese and Indian manufacturers are considered as suppliers of medicines, raw materials for their manufacture, feed additives [4].
For the record:
Among the foreign pharmaceutical companies present on the Russian market, there is still not a single company that has completely localized the production of its veterinary medicines. But even if a company has outsourced the production of at least a few medicines, it may be easier for such a foreign manufacturer to prepare for a Russian inspection. Organizing Contract Manufacturing Organization (CMO) audits, participating in a regular Quality Review of manufactured medicinal products and jointly evaluating the results of such a review is a good practice that helps to better understand local requirements.
Russian manufacturers
According to the register on the Rosselkhoznadzor website [1], in the second half of the current year, 11 GMP-conclusions were issued. These conclusions were obtained by the following manufacturing sites: Stavropol Biofactory, Apicenna, Agrovetzashchita S-P, I. Ya. Postovsky Institute of Organic Synthesis, Eliksir-D, Vetlain, Ekoprom, Moscow Endocrine Plant, DEKO-PHARM, Armavir Biofactory and Astrapharm. In total, 14 conclusions were issued to domestic manufacturers in 2022, which is more than in the previous year.
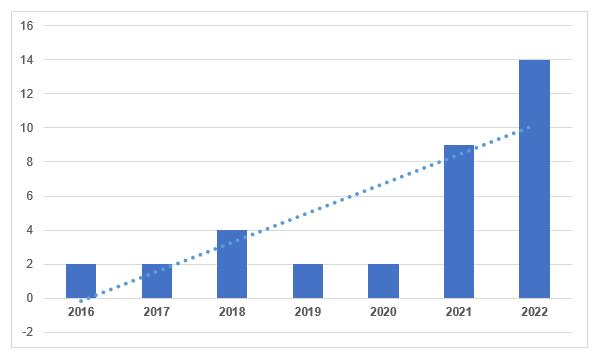
To date, domestic manufacturers have 25 valid conclusions – almost the same number as foreign manufacturers.
For the record:
According to Rosselkhoznadzor, 101 local manufacturers are currently operating on the veterinary medicines market in the Russian Federation. By 2030, domestic manufacturers intend to increase the volume of production of Russian veterinary medicines by 5 times compared to 2021, from 24 to 100 billion rubles, and occupy 90% of the domestic market, but subject to state support. Such indicators are spelled out in the draft concept for the development of the veterinary medicines production industry in Russia. The document was prepared by a working group of the National Veterinary Association (NVA). Since March 2022, due to the entry into force of amendments to Federal Law No. 99-FZ dated 04.05.2011 “On Licensing Certain Types of Activities“ [5], Russian manufacturers can receive GMP-conclusions based on the results of periodic confirmation of compliance with licensing requirements, which replaced scheduled inspections.
You can read more about domestic manufacturers of veterinary medicines here (RU).
In the first half of this year, some media expressed doubts that the Russian inspectorate could carry out a sufficient number of inspections. For example, in one of its publications, the RTVI news portal referred to the opinion of Nikolay Bespalov, Development Director of the analytical company RNC Pharma, who noted that, in addition to problems with on-site inspections at enterprises in unfriendly countries, there is a lack of specialists themselves [7].
However, in the second half of the year, the Russian inspectorate began to conduct significantly more inspections. For example, in November, information was published on the FSBI “VGNKI” website that from September 26 to November 10, specialists from the Inspection Department conducted 10 inspections of foreign manufacturers of medicinal products (including one visit to the manufacturing site and nine inspections on documents, including in a remote format using audio or video communication) [8]. This result (10 inspections in 1.5 months) confirms in practice that the current forces of the inspectorate can carry out at least 80 inspections per year.
For the record:
For various reasons, some manufacturers themselves ask to postpone inspections of their sites to a later date. At the same time, it should be remembered that the inspection must be carried out within a period not exceeding 160 working days from the date of the decision by the authorized body to conduct an inspection (in accordance with paragraph 20. of Decree of the Government of the Russian Federation dated 03.12.2015 No. 1314 [9]).
Past events and activities
In the second half of 2022, several events took place in Russia that could be useful for manufacturers to prepare for the inspection.
This year, the list of training events of the Scientific and Methodological Base Center of the FSBI “VGNKI” included 95 programs, including 3 new programs for employees of enterprises manufacturing medicinal products for veterinary use [10].
In particular, an online seminar on the specifics of the production of biological pharmaceutical substances and medicines was held in July. During the webinar, specialists from the GMP Inspection Department told the audience about the special requirements for different types of products, raw materials, packaging materials and biological production personnel, as well as about the design features of premises and equipment [11].
In September, the VII All-Russian GMP Conference with international participation, organized by the Ministry of Industry and Trade of Russia together with the FSI “SID & GP”, was held in Irkutsk [12].
Within the framework of the Conference, a round table of the Association of Veterinary Pharmaceutical Manufacturers AVFARM “GMP of veterinary pharmaceutical industries: trends and prospects” was held. This event brought together the heads of inspection bodies of the Russian Federation and the Republic of Belarus, representatives of the Eurasian Economic Commission (EEC) and business, as well as Russian and international experts in the field of GMP Rules. The main topics of discussion were changes in the mechanism for conducting inspections in connection with the entry into force of the new “Rules for the regulation of the circulation of veterinary medicinal products in the customs territory of the EAEU”, approved by the Resolution of the EEC Council dated 21.01.2022 No. 1 [13], as well as the dynamics and prospects of inspections conducted by Russian and Belarusian authorized bodies [14].
During the round table, the Head of the Inspection Body of the FSBI “VGNKI” Yuri Eremin, spoke about the accumulated experience of conducting remote inspections. He noted that 12 inspectors currently work in the GMP Inspection Department, which can carry out up to 80 inspections per year [15].
Yuri Eremin specifically dwelled on the difficulties that arise when submitting to the authorized institution a plan of corrective and preventive actions (CAPA-plan) and copies of documents containing measures for its implementation (in accordance with clause 27 of Decree of the Government of the Russian Federation dated 03.12.2015 No. 1314 [9]):
- incomplete translation of documents; translation carried out by an unqualified interpreter;
- supporting primary records are not provided; often only draft protocols and reports are provided.
The Head of the Inspection Body also drew attention to the fact that the websites of Rosselkhoznadzor and the FSBI “VGNKI” posted “Methodological recommendations for preparing for a remote inspection of a foreign manufacturer of medicinal products for veterinary use” [16, 17], which should be followed when preparing for a remote inspection.
For the record:
Interpreters who will be translating CAPA-plan related documents need to be provided with the additional information they need to perform services in a professional manner. Some translation agencies separately stipulate in the contract the provision of a terminological glossary or other reference and information materials. This can be, for example, a list of abbreviations used in documents, previously translated texts with source files, translation memory files, etc. In order to translate all documents related to the CAPA-plan in a timely manner, it is possible to begin work on the preparation of some of these documents even during the preparation of the initial inspection report – focusing on the observations that were voiced at the closing meeting on the last day of the inspection.
Deputy Director of the Department for Sanitary, Phytosanitary and Veterinary Measures of the EEC Vladimir Subbotin made a presentation on the topic “Application of the GMP EAEU requirements in the context of new rules for the regulation of the circulation of veterinary medicinal products in the EAEU”. In addition, he answered questions from the round table participants:
Question 1: Will the registration procedures in the EAEU Member States require confirmation of GMP compliance with respect to specific medicinal products/dosages or only in relation to dosage forms/production lines?
Answer: The inspections are carried out and documents are issued in relation to specific manufacturing sites, and if they are large enough, then with regard to individual production lines. If there is a production of several medicinal products on these lines or sites, all of them fall under the possibility of registration based on these GMP Certificates issued.
Question 2: Will the issued GMP Certificate be mutually recognized in two (or more) EAEU Member States if the inspectors of only one authorized body of the EAEU Member State, from those Member States in whose territory it is planned to circulate the medicinal products participated in the inspection of manufacturer?
Answer: Yes, the Certificate issued by one specific Authorized Body for compliance with the GMP rules of the Union will be recognized in other Member States.
GMP expert of veterinary pharmaceutical industries Emmanuelle Motte presented the HealthforAnimals association. Among the areas of international cooperation, she mentioned mutual recognition agreements, as well as participation in international organizations such as the Pharmaceutical Inspection Co-operation Scheme, PIC/S. It should be mentioned that on the same day of the GMP Conference, Vladislav Shestakov, Director of the FSI “SID & GP”, moderating the plenary session “International cooperation in the field of Good Practices in modern geopolitical conditions”, said that Russia’s application to join the PIC/S was frozen [19].
Commenting on the negative results of Russian inspections of sites of manufacturers of immunobiological medicines in the USA, Danil Rudniaev, Deputy Director of the FSBI “VGNKI”, noted that American sites need to study the EAEU GMP Rules, approved by the Resolution of the EEC Council dated 03.11.2016 No. 77 [20], and conduct audits at the sites for compliance with the requirements of these Rules and not just those requirements that the United States Department of Agriculture (USDA) imposes. “We need to look at what is wrong, what needs to be corrected in relation to those medicines that will be submitted for the Russian inspection, and only after that submit an application,” said Danil Rudniaev.

For the record:
The “List of the most common nonconformities when inspecting manufacturers of medicinal products for veterinary use” [21] was first posted on FSBI “VGNKI” website in 2018, and it is updated periodically. This information must be used in preparation for the inspection. Preliminary audit, study of reports on the results of recent inspections, control over the CAPA plan implementation, as well as the study of individual issues related to the upcoming Russian inspection, are very important in preparation. It is important that a pre-audit be carried out at all manufacturer sites that are to be inspected, including CMO sites.
In addition, the following speakers spoke at the round table: Head of the Department of Pharmaceutical Inspection of the SI “Belarusian State Veterinary Center” Nina Malash, Head of Section for Drug manufacturing inspections of the FSI “SID & GP” Natalia Popova, Executive Director of the AVFARM Association Semen Zhavoronkov and Executive Director of the NVA Association Timur Chibilyaev.
Many participants joined the round table online. For representatives of French organizations, participation in this event is important and already traditional, as it provides an opportunity to maintain and deepen international cooperation in the field of quality assurance and quality control of veterinary medicines. Among the participants was Marie-Agnès Amos, Deputy Adviser for Agriculture, Assistant for Veterinary, Sanitary, Phytosanitary Issues for Russia, Kazakhstan, Belarus, Armenia, Uzbekistan, Kyrgyzstan of the Economic Service of the French Embassy in Russia.
In October, Moscow hosted the IV International Conference “Medicinal products logistics” [22]. This annual Сonference may be of interest to manufacturers of veterinary medicines already because in September 2022, on the federal portal of draft regulatory legal acts (https://regulation.gov.ru/) a draft Order of the Ministry of Agriculture of Russia “On Approval of the Rules for Good Distribution Practice of medicinal products for veterinary use” was posted for public discussion [23]. If the document is approved, it will enter into force on September 1, 2023 and will be valid until March 12, 2024.
At the Сonference, Elena Zelinskaya (Quality Director, Pharma Capital) made a presentation on the topic “Harmonization of deficiencies classifications”. She specifically focused on the PIC/S document PI 040-1 “Guidance on Classification of GMP Deficiencies”, the purpose of which is to harmonize classifications of deficiencies and ensure consistency between inspections [24].
All participants of the Conference were presented by the Community of Managers and Specialists in Logistics and Quality SCM Pharm with the Collection of Practical Articles GDP Review 3. Among the materials presented in the collection, one can note the article “Conducting remote GMP inspections” (Evgeny Gladyshev, Senior Manager, International Quality and Distribution, Amgen and Yulia Stepanova, Manager, International Quality and Distribution, Amgen). The authors of the article note that virtual inspections have a number of limitations, take more time and, accordingly, require careful preparation on the part of the Applicant and the manufacturer. An important role during the preparation and conduct of the inspection is assigned to IT specialists: it is necessary to ensure unhindered communication and organize online broadcasts. The effectiveness and success of an inspection using remote interaction tools depends, among other things, on the quality and level of information technology used. Key elements to consider when preparing for an inspection, in addition to technical feasibility, are the safety and privacy of remote connections. It is necessary to work out in advance possible legal problems and requests for the implementation of video recordings and the provision of documentation on electronic media. Secure pathways for document exchange and file transfer should be identified, agreed upon and established. It is recommended to carry out preliminary testing of access to information systems for video communication and exchange of documents in advance, before the start of the inspection.
Manufacturers of medicines need to keep an eye on the information released by regulators and industry organizations and be prepared for any type of inspection. In one of the October publications on the “Zooinform” portal [25], it is noted that the remote inspections currently being carried out are not so simple: seamless Internet coverage is required throughout the site, a larger volume of documents for translation, there may be a critical difference in time zones and etc.
For the record:
According to the “Methodological recommendations for preparing for a remote inspection of a foreign manufacturer of medicinal products for veterinary use” prepared by the FSBI “VGNKI”, for remote analysis, the site can provide inspectors with both live camera footage and video recordings. Clause 2.4 of the European Medicines Agency document EMA/335293/2020 “Guidance related to GMP/GDP and PMF distant assessments” also talks about the ability to provide live camera footage or video recordings [26]. Please note that video recordings that are too long can create additional problems during a video conference, especially if the Internet connection is unstable. Some handwritten documents (such as records, logbooks, etc.) can be demonstrated using a document camera – a special video equipment on a tripod that allows you to get a clear image of any objects and broadcast it on the screen in real time. For some reason, some manufacturers do not think about the need to order translation of site documents in advance. In accordance with the conventional norms for the workload of interpreters, a professional interpreter can translate approximately 6-10 pages of text per day (if we take a standard page equal to 1800 printed characters or 250 words as a unit of account). It is this number of pages that is quite realistic to translate during the working day at a decent level of quality. This should be taken into account when planning the timing of the translation of documentation for inspection.
You can also read about preparation for remote inspection here (RU).

In October, a business meeting was held between Irina Spichak, Executive Director of the Eurasian Academy of Good Practices, and Danil Rudniaev, Deputy Director of the FSBI “VGNKI”. During the meeting, current trends in industry education were discussed, as well as issues of cooperation between organizations, in particular, the prospects for using the Academy’s VR plant for the FSBI “VGNKI” inspectorate [27].
Simulation virtual pharmaceutical complex “Virtual Plant 2.0” is a unique product based on virtual reality technologies, designed to develop the skills of inspecting the production of medicinal products for compliance with GMP standards. The user of the system has the opportunity to go through all stages of a real inspection of a pharmaceutical plant of solid forms, starting from arrival at the enterprise and meeting with employees and ending with a complete inspection of production premises and equipment. The hardware part of the system is a set consisting of a personal computer with installed software, virtual reality glasses and controllers for control. When using the simulator, the image from the VR glasses can be broadcast to an external TV screen or projector, thus allowing the curator or a large audience of spectators to observe what is happening [28].
In the second half of 2022, a series of free webinars was held on the Academy platform as part of the program of additional professional education. For example, in August, a webinar was held on the topic “Deviation management and CAPA. OOS/OOT/OOE”, in September – on the topic “Ensuring the data integrity in the medicines production processes”, in October – on the topic “Fundamentals of validation of computerized systems” [29, 30].
Among the new educational projects for specialists of pharmaceutical companies, the GxP Training Center can be noted.
In October, the Training Center held a free webinar on the topic “Area of responsibility of Qualified Persons of a medicinal product manufacturer. Who is responsible for quality?“ Among the questions that were asked during the webinar was the question of the requirements that apply to Qualified Persons. From the response received, one can see the difference in the requirements for practical experience between manufacturers of medicinal products for human and veterinary use [31]. In accordance with the “Procedure for certification of Qualified Persons of medicinal product manufacturers”, approved by the Resolution of the EEC Council dated 03.11.2016 No. 73 [32], the certified Qualified Person must have at least 3 years of work experience in the field of production, or quality assurance, or quality control of medicinal products. According to the “Procedure for certification of a Qualified Person of a manufacturer of medicinal products for veterinary use”, approved by Order of the Ministry of Agriculture of Russia dated 22.10.2021 No. 720 [33], which will enter into force on March 1, 2024, employees of manufacturers of medicinal products for veterinary use with at least 5 years of work experience in the field of production and (or) quality control of medicinal products will be allowed to be certified.
For the record:
Work experience of at least 5 years in the field of production and (or) quality control of medicinal products or in the field of monitoring the efficacy and safety of medicinal products for veterinary use will also be required in accordance with the new “Procedure for certification of a Person authorized by the holder or owner of the marketing authorization of a medicinal product for veterinary use”, approved by Order of the Ministry of Agriculture of Russia dated 10.11.2022 No. 795 [34]. In this case, the certified persons will have to pass a test control of knowledge, which provides for the performance of test tasks within 120 minutes. The test tasks include 50 questions concerning the requirements of the GMP Rules, as well as the requirements for the registration dossier for a medicinal product for veterinary use. Within the time allotted for the test control of knowledge, it is impossible for certified persons to use regulatory legal acts, reference literature (in paper form or on electronic media) – it is not allowed to use reference literature, written notes, it is impossible to use mobile communications, photo, audio and video equipment. In accordance with Part 2 of Article 52.2 of Federal Law No. 61-FZ of 12.04.2010 “On the Circulation of Medicines”, introduced by Federal Law No. 317-FZ of 02.07.2021 [35], before entering into civil circulation of each batch of a medicinal product for veterinary use imported into the Russian Federation, the importing organization will have to submit to Rosselkhoznadzor a confirmation of the compliance of the medicinal product with the requirements established during its state registration, from the Person authorized by the holder or owner of the marketing authorization of a medicinal product for veterinary use. At the moment, there is no such guidance on confirmation of the compliance (in contrast to the guidance on the certification by a Qualified Person, which is established by Annex 16 to the EAEU GMP Rules, approved by the Resolution of the EEC Council dated 03.11.2016 No. 77 [20]).
For objective reasons, not all planned events took place in 2022. For example, the ISPE EAEU Annual Conference planned in St. Petersburg was postponed to 2023. As it is written on the website of the Eurasian branch of the association, the strength of such events is the cross-country and interregional coverage of key problems and aspects of the development of the pharmaceutical industry, but in the current conditions this aspect is serious is reduced [36].
However, it was possible to take part in some international events remotely. For example, the free webinar “Annex 1 – what it means for you”, organized in October by the French company Elis and dedicated to the new Annex 1 to EU GMP [37], was very informative. This Annex will be implemented in the European Union in August 2023. As Leading Qualification Specialist of PQE Group Alexander Belinsky recently noted, without constant communication and training, knowledge becomes obsolete. The new Annex, dedicated to the manufacture of sterile medicinal products, contains a lot of new information, new approaches and regulatory requirements, which, with minimal changes, will most likely be introduced in EAEU [38].
In December, it was possible to take part in a free webinar “Approaches for pharmaceutical supplier qualification” held by the Indian company Pharmazone. The webinar addressed issues such as the need for valuation, the type and mode of evaluation, applicable guidance, and challenges in supplier qualification. The topic of qualification of material suppliers and service providers is very important. For example, in 2019, the following nonconformity was included in the “List of the most common nonconformities when inspecting manufacturers of medicinal products for veterinary use” – outsourcing activities are not controlled.
Challenges in 2023
During an interview with Federal specialized publication “Veterinary Medicine and Life”, the Director of FSBI “VGNKI” Leonid Kish drew attention to the fact that from September 1, 2023, amendments to Federal Law No. 61-FZ of 12.04.2010 “On the Circulation of Medicines”, introduced by Federal Law No. 317-FZ of 02.07.2021 [35], come into force, and those companies (mostly foreign) that do not comply with the GMP standard will not be able to sell their medicines on the territory of the Russian Federation [39].
In an interview with the same Federal specialized publication, the Executive Director of the AVFARM Association Semen Zhavoronkov said that international companies, as before, operate on the Russian market, and Russian teams are now making every effort to fulfill their obligations to consumers despite sanctions restrictions and logistical difficulties, as well as to comply with legal requirements [40].
In September 2022, the AVPHARM Association launched an open data section for all current and potential new industry participants, where a register of domestic and foreign manufacturing sites that have been certified for compliance with the GMP Rules requirements is published [41]. This register is a simplified version of the register of GMP-conclusions on the Rosselkhoznadzor website [1], but it does not contain a list of medicinal products for veterinary use produced by the site. It should also be borne in mind that the production and quality control process of some medicines can be shared among several manufacturing sites that perform separate stages.
As of the end of the outgoing year, foreign manufacturers have 26 valid GMP-conclusions, the number of which has not increased for the second year in a row (at the beginning of 2021, foreign manufacturers had 37 valid conclusions).
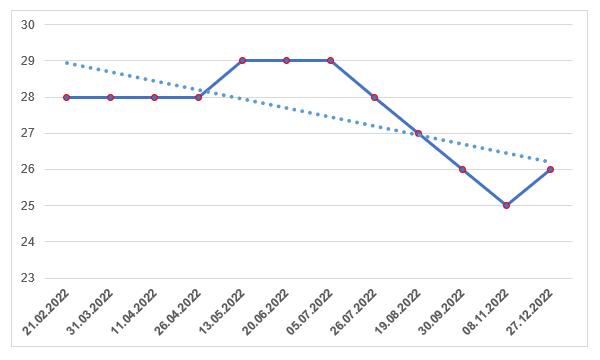
This situation cannot but worry foreign manufacturers of veterinary medicines, since only eight months are left until September next year, during which it is required to obtain a GMP-conclusion. Therefore, in 2023, we can expect an increase in the number of inspections of manufacturers of medicinal products for veterinary use, in contrast to manufacturers of medicinal products for human use, which are expected to have a decrease in the number of inspections in the next two years [42].
In accordance with the inspection schedule published on the Rosselkhoznadzor website 27.12.2022 [43], 33 inspections of manufacturers of veterinary medicines are planned for 2023, which sites located in Australia, Austria, Belgium, Brazil, Hungary, Germany, Spain, Italy, China, the Netherlands, New Zealand, Romania, the USA, France, Czech Republic and Estonia. Most of the inspections (7) should take place in relation to the manufacturing sites located in France.
Manufacturers are encouraged to take an active part in GM(D)P-related events and activities and prepare more thoroughly for GMP inspections.
The material presented was prepared using data relevant to 27.12.2022. In case of new or additional data the article can be updated.
***
The Federal State Budgetary Institution “The All-Russian State Center for Quality and Standardization of Veterinary Drugs and Feed”, FSBI “VGNKI” is an institution authorized to conduct inspections of manufacturers of medicinal products for veterinary use manufactured outside the Russian Federation for compliance with the GMP rules requirements
The Federal State Institution “State Institute of Drugs and Good Practices”, FSI “SID & GP” is an institution authorized to conduct inspections of manufacturers of medicinal products for human use manufactured outside the Russian Federation for compliance with the GMP rules requirements
The State Institution “Belarusian State Veterinary Center” is an institution of the Republic of Belarus authorized to conduct pharmaceutical inspections of the production of veterinary medicinal products for compliance with the GMP rules requirements
Agence Nationale du Médicament Vétérinaire, ANMV is the French Agency for Veterinary Medicinal Products. The Agency monitors the quality of medicinal products for veterinary use distributed in France. In addition, ANMV issues marketing authorizations for the veterinary medicinal products, the licensing of pharmaceutical sites for the manufacturing, distribution and export
The National Food Chain Safety Office, NÉBIH is the integrated food safety authority of Hungary. NÉBIH is responsible for the control of the whole food chain from farm to fork, including soil protection, agricultural production, forestry, food processing, retail and catering
The European Medicines Agency, EMA is an agency of the European Union in charge of the evaluation and supervision of medicinal products
The United States Department of Agriculture, USDA. The functions of the department include the implementation of agricultural and food policies, including food safety, rural development, financing of agricultural research. USDA inspects manufacturers of immunobiological medicinal products for veterinary use on the territory of the USA
The Association for the Promotion of the Development of Veterinary Affairs “National Veterinary Association”, NVA is an association that unites the largest domestic manufacturers of veterinary drugs
The Association of Veterinary Pharmaceutical Manufacturers AVFARM is an association representing the interests of leading international pharmaceutical companies in the Russian Federation – manufacturers of medicinal products for veterinary use (MSD Animal Health, Elanco, Zoetis and Boehringer Ingelheim)
HealthforAnimals is a non-profit, non-governmental organization representing companies and industry associations from developed and developing countries (manufacturers of veterinary pharmaceuticals, vaccines and other animal health products throughout the world, as well as the associations that represent companies at national and regional levels)
The Pharmaceutical Inspection Co-operation Scheme (PIC/S) is a non-binding, informal co-operative arrangement between Regulatory Authorities in the field of GMP of medicinal products for human or veterinary use
Eurasian branch of ISPE, ISPE EAEU is a local branch of ISPE in the Eurasian Economic Union, created to provide expert support for the development of Good Practices in the pharmaceutical industry on the territory of the EAEU
REFERENCES:
- Россельхознадзор / Деятельность / Госуслуги / Выдача заключения о соответствии производителя (иностранного производителя) лекарственных средств для ветеринарного применения требованиям правил надлежащей производственной практики / Дополнительная информация для заявителя / Государственный реестр заключений о соответствии производителя (иностранного производителя) лекарственных средств для ветеринарного применения требованиям правил надлежащей производственной практики. URL: https://fsvps.gov.ru/ru/gosuslugi/zaklyuchenie-o-sootvetstvii (дата обращения 12.2022)
- KRKA / КРКА в России / Завод OOO «КРКА-РУС». URL: https://www.krka.biz/ru/krka-v-rossii/predstavleniye/ooo-krka-rus/ (дата обращения 12.2022)
- RNC Pharma / Новости / RNC Pharma представляет информацию относительно активности ввоза ветеринарных ЛП и кормовых добавок в Россию по итогам октября 2022 г. URL: https://rncph.ru/news/30_11_2022 (дата обращения 27.12.2022)
- Россельхознадзор / Новости / Главные новости / Сергей Данкверт принял участие в заседании Общественного совета при Россельхознадзоре. URL: https://fsvps.gov.ru/ru/fsvps/news/47946.html (дата обращения 27.12.2022)
- Ветеринария и жизнь / Зообизнес / Производство российских ветпрепаратов увеличат в 5 раз к 2030 году. URL: https://vetandlife.ru/sobytiya/proizvodstvo-rossijskih-vetpreparatov-uvelichat-v-5-raz-k-2030-godu/ (дата обращения 27.12.2022)
- Электронный фонд правовых и нормативно-технических документов / Федеральный закон от 04.05.2011 г. № 99-ФЗ «О лицензировании отдельных видов деятельности» (редакция, действующая с 1 марта 2022 года). URL: https://docs.cntd.ru/document/902276657 (дата обращения 27.12.2022)
- RTVI / Новости / Производители иностранных ветпрепаратов предупредили об угрозе сокращения импорта в Россию на 85%. URL: https://rtvi.com/news/proizvoditeli-inostrannyh-vetpreparatov-predupredili-ob-ugroze-sokrashheniya-importa-v-rossiyu-na-85/ (дата обращения 27.12.2022)
- ФГБУ «ВГНКИ» / Главная / Пресс-центр / Новости / Специалисты отдела инспекции ФГБУ «ВГНКИ» провели инспектирования производителей лекарственных средств для ветеринарного применения. URL: https://www.vgnki.ru/specialisty-otdela-inspekcii-fgbu-vgnki-proveli-inspektirovaniya-proizvoditelej-leksredstv-dlya-veterinarnogo-primeneniya.html (дата обращения 27.12.2022)
- ФГБУ «ВГНКИ» / Структура / Отдел инспекции производства на соответствие требованиям надлежащей производственной практики / Нормативно-правовая документация / Постановление Правительства РФ от 03.12.2015 N 1314 (ред. от 05.09.2020) «Об определении соответствия производителей лекарственных средств требованиям правил надлежащей производственной практики» (вместе с «Правилами организации и проведения инспектирования производителей лекарственных средств на соответствие требованиям правил надлежащей производственной практики, а также выдачи заключений о соответствии производителя лекарственных средств указанным требованиям»). URL: http://www.vgnki.ru/assets/files/post-1314.pdf (дата обращения 27.12.2022)
- ФГБУ «ВГНКИ» / Пресс-центр / Новости / Итоги работы отдела «Научно-методический базовый центр» за 2022 год. URL: https://www.vgnki.ru/itogi-raboty-otdela-nauchno-metodicheskij-bazovyj-centr-za-2022-god.html (дата обращения 27.12.2022)
- ФГБУ «ВГНКИ» / Главная / Пресс-центр / Новости / В ФГБУ «ВГНКИ» прошел вебинар по специфике производства лекарственных средств для животных. URL: https://www.vgnki.ru/v-fgbu-vgnki-proshel-vebinar-po-specifike-proizvodstva-lekarstvennyh-sredstv-dlya-zhivotnyh.html (дата обращения 27.12.2022)
- VII Всероссийская GMP-конференция с международным участием / Программа. URL: https://gosgmp.ru/download/Programma-Konferentsii-2022.pdf (дата обращения 27.12.2022)
- Евразийский экономический союз / Решение Совета Евразийской экономической комиссии от 21.01.2022 № 1 «О Правилах регулирования обращения ветеринарных лекарственных средств на таможенной территории Евразийского экономического союза. URL: https://docs.eaeunion.org/Pages/DisplayDocument.aspx?s=%7Be1f13d1d-5914-465c-835f-2aa3762eddda%7D&w=9260b414-defe-45cc-88a3-eb5c73238076&l=%7B8a412e96-924f-4b3c-8321-0d5e767e5f91%7D&EntityID=32004 (дата обращения 27.12.2022)
- АВФАРМ / Новости / Результаты и перспективы прохождения инспекций на соответствие GMP ЕАЭС ветеринарными фармацевтическими производителями обсудили на конференции в Иркутске. URL: https://avpharm.ru/news/rezultaty-i-perspektivy-prokhozhdeni/ (дата обращения 27.12.2022)
- Союз предприятий зообизнеса / Новости / Российский инспекторат может проводить 80 инспекций в год. URL: http://www.spzoo.ru/cntnt/default/n7609.html (дата обращения 27.12.2022)
- Россельхознадзор / Деятельность / Госуслуги / Выдача заключения о соответствии производителя (иностранного производителя) лекарственных средств для ветеринарного применения требованиям правил надлежащей производственной практики / Дополнительная информация для заявителя / Методические рекомендации по подготовке к дистанционной инспекции иностранного производителя лекарственных средств для ветеринарного применения. URL: https://fsvps.gov.ru/sites/default/files/files/gosuslugi-lek/guid_for_preparing_for_inspection.pdf (дата обращения 27.12.2022)
- ФГБУ «ВГНКИ» / Структура / Отдел инспекции производства на соответствие требованиям надлежащей производственной практики / Полезная информация / Методические рекомендации по подготовке к дистанционной инспекции иностранного производителя лекарственных средств для ветеринарного применения. URL: https://www.vgnki.ru/otdel-inspekcii-proizvodstva-na-sootvetstvie-trebovaniyam-nadlezhashhej-proizvodstvennoj-praktiki.html (дата обращения 27.12.2022)
- Союз предприятий зообизнеса / Новости / Ответы на вопросы производителей на круглом столе «GMP ветеринарных фармацевтических производств: тенденции и перспективы». URL: http://www.spzoo.ru/cntnt/default/n7601.html (дата обращения 27.12.2022)
- ФармПром.РФ / Регуляторы фармрынка / Владислав Шестаков: Заявка России о вступлении в членство Схемы PIC/S была заморожена. URL: https://pharmprom.ru/vladislav-shestakov-zayavka-rossii-o-vstuplenii-v-chlenstvo-sxemy-pic-s-byla-zamorozhena/ (дата обращения 27.12.2022)
- Евразийский экономический союз / Решение Совета Евразийской экономической комиссии от 03.11.2016 г. № 77 «Об утверждении Правил надлежащей производственной практики Евразийского экономического союза». URL: https://docs.eaeunion.org/docs/ru-ru/01411921/cncd_21112016_77 (дата обращения 27.12.2022)
- ФГБУ «ВГНКИ» / Структура / Отдел инспекции производства на соответствие требованиям надлежащей производственной практики / Полезная информация / Перечень наиболее часто встречающихся несоответствий при инспектировании производителей лекарственных средств для ветеринарного применения. URL: https://www.vgnki.ru/otdel-inspekcii-proizvodstva-na-sootvetstvie-trebovaniyam-nadlezhashhej-proizvodstvennoj-praktiki.html (дата обращения 27.12.2022)
- SCM Pharm / Мероприятия / IV Международная конференция: Логистика лекарственных средств / Конференция / Программа конференции. URL: https://scmpharm.ru/events/4-offline-conf/ (дата обращения 27.12.2022)
- Федеральный портал проектов нормативных правовых актов / Проект Приказа Минсельхоза России «Об утверждении Правил надлежащей дистрибьюторской практики лекарственных препаратов для ветеринарного применения». URL: http://regulation.gov.ru/p/131144 (дата обращения 12.2022)
- Pharmaceutical Inspection Co-operation Scheme / PIC/S Guidance on Classification of GMP Deficiencies (PI 040-1). URL: https://picscheme.org/docview/2303 (дата обращения 12.2022)
- Зооинформ / Статьи / Рынок зоотоваров / Ненадлежащее регулирование надлежащей практики. URL: https://zooinform.ru/business/articles/nenadlezhashhee-regulirovanie-nadlezhashhej-praktiki/ (дата обращения 27.12.2022)
- EMA / GMP/GDP Inspectors Working Group / Guidance related to GMP/GDP and PMF distant assessments. URL: https://www.ema.europa.eu/en/documents/scientific-guideline/guidance-related-gmp/gdp-pmf-distant-assessments_en.pdf (дата обращения 27.12.2022)
- Евразийская Академия надлежащих практик / Об Академии / Новости и события / 18 октября прошла деловая встреча исполнительного директора Академии Ирины Спичак и замдиректора ФГБУ «ВГНКИ» Данила Рудняева. URL: https://gxp-academy.org/about/news_and_events/1020/ (дата обращения 27.12.2022)
- Симуляционный виртуальный фармацевтический комплекс GxP. URL: https://vrzavod.org/ (дата обращения 27.12.2022)
- Евразийская Академия надлежащих практик / Об Академии / Новости и события / Евразийская Академия надлежащих практик приглашает 10 августа на бесплатный вебинар «Управление отклонениями и САРА. OOS/OOT/OOE». URL: https://gxp-academy.org/about/news_and_events/699/ (дата обращения 27.12.2022)
- Евразийская Академия надлежащих практик / Об Академии / Новости и события / Евразийская Академия надлежащих практик совместно с холдингом «Нацимбио» проводит серию бесплатных вебинаров. URL: https://gxp-academy.org/about/news_and_events/1019/ (дата обращения 27.12.2022)
- ФармПром.РФ / Фармацевтическое образование / Кто несет ответственность за качество? Ответы на часто задаваемые вопросы. URL: https://pharmprom.ru/kto-neset-otvetstvennost-za-kachestvo-otvety-na-chasto-zadavaemye-voprosy/ (дата обращения 27.12.2022)
- Евразийский экономический союз / Решение Совета Евразийской экономической комиссии от 03.11.2016 г. № 73 «О Порядке аттестации уполномоченных лиц производителей лекарственных средств». URL: https://docs.eaeunion.org/docs/ru-ru/01413885/cncd_21112016_73 (дата обращения 27.12.2022)
- Электронный фонд правовых и нормативно-технических документов / Приказ Министерства сельского хозяйства Российской Федерации от 22.10.2021 № 720 «Об утверждении Порядка аттестации уполномоченного лица производителя лекарственных средств для ветеринарного применения». URL: https://docs.cntd.ru/document/727092847 (дата обращения 27.12.2022)
- Официальный интернет-портал правовой информации / Приказ Министерства сельского хозяйства Российской Федерации от 10.11.2022 № 795 «Об утверждении Порядка аттестации лица, уполномоченного держателем или владельцем регистрационного удостоверения лекарственного препарата для ветеринарного применения». URL: http://publication.pravo.gov.ru/Document/View/0001202211290041 (дата обращения 27.12.2022)
- Электронный фонд правовых и нормативно-технических документов / Федеральный закон от 02.07.2021 г. № 317-ФЗ «О внесении изменений в Федеральный закон «Об обращении лекарственных средств». URL: https://docs.cntd.ru/document/607142404 (дата обращения 27.12.2022)
- ISPE ЕАЭС / Конференция ISPE ЕАЭС / Новости / Изменение дат III Ежегодной Конференции ISPE ЕАЭС. URL: https://conference.ispe.ru/ (дата обращения 27.12.2022)
- The rules governing medicinal products in the European Union – Volume 4 – EU Guidelines for Good Manufacturing Practice for Medicinal Products for Human and Veterinary Use / Annex 1 – Manufacture of Sterile Medicinal Products. URL: https://health.ec.europa.eu/system/files/2022-08/20220825_gmp-an1_enpdf (дата обращения 27.12.2022)
- А. Тихонова. Непрерывное обучение, актуализация знаний и обмен опытом // ФАРМПРОМ, 2022, № 2. URL: https://pharmprom.ru/farmprom-otraslevoj-zhurnal-onlajn-vypusk-2-2022/ (дата обращения 27.12.2022)
- Ветеринария и жизнь / Главная / Интервью / Гость студии «Ветеринарии и жизни» – директор ФГБУ «ВГНКИ» Леонид Киш. URL: https://vetandlife.ru/sobytiya/gost-studii-veterinarii-i-zhizni-direktor-fgbu-vgnki-leonid-kish/ (дата обращения 27.12.2022)
- Ветеринария и жизнь / Главная / Интервью / Глава АВФАРМ: ассоциация планирует объединить международные фармацевтические компании стран Запада и Востока. URL: https://vetandlife.ru/sobytiya/glava-avfarm-associaciya-planiruet-obedinit-mezhdunarodnye-farmacevticheskie-kompanii-stran-zapada-i-vostoka/ (дата обращения 27.12.2022)
- АВФАРМ / Аналитика / Предприятия с GMP. URL: https://analytics.avpharm.ru/gmp (дата обращения 27.12.2022)
- Фармацевтический вестник / Новости / Производство / ГИЛС ожидает снижение количества зарубежных инспекций. URL: https://pharmvestnik.ru/content/news/GILS-ojidaet-snijenie-kolichestva-zarubejnyh-inspekcii.html (дата обращения 27.12.2022)
- Россельхознадзор / Деятельность / Госуслуги / Выдача заключения о соответствии производителя (иностранного производителя) лекарственных средств для ветеринарного применения требованиям правил надлежащей производственной практики / Дополнительная информация для заявителя / График инспектирования производителей. URL: https://fsvps.gov.ru/ru/gosuslugi/zaklyuchenie-o-sootvetstvii (дата обращения 27.12.2022)
