The review provides information related to the inspection of manufacturers of medicinal products for veterinary use for compliance with the requirements of the Good Manufacturing Practice (GMP). These inspections are carried out by specialists of the Federal State Budgetary Institution “The All-Russian State Center for Quality and Standardization of Veterinary Drugs and Feed” (FSBI “VGNKI”) subordinated to Rosselkhoznadzor.

Inspection results
Since the beginning of 2021, the inspection of manufacturers of medicinal products for veterinary use has been carried out for compliance with the requirements of the GMP Rules of the Eurasian Economic Union (EAEU GMP Rules), approved by the Resolution of the EEC Council No. 77 of November 3, 2016 [1].
Over the past year, the specialists of the Department of Inspection of Manufacture for Compliance with the Requirements of the Good Manufacturing Practice conducted 32 inspections of foreign manufacturers, including 17 on-site and 15 in a remote format. In addition, the specialists of the department, as experts, took part in 83 inspections of Russian manufacturers carried out by Rosselkhoznadzor, of which 23 scheduled inspections and 55 unscheduled [2].
According to the register of issued conclusions published on the Rosselkhoznadzor website 28.12.2021, in 2021, foreign manufacturers were issued 11 conclusions on compliance with the GMP Rules requirements. 8 GMP-conclusions were issued to Russian manufacturers [3].
As of the end of 2021, foreign manufacturers had 28 valid GMP-conclusions; Russian manufacturers had 12 valid conclusions.
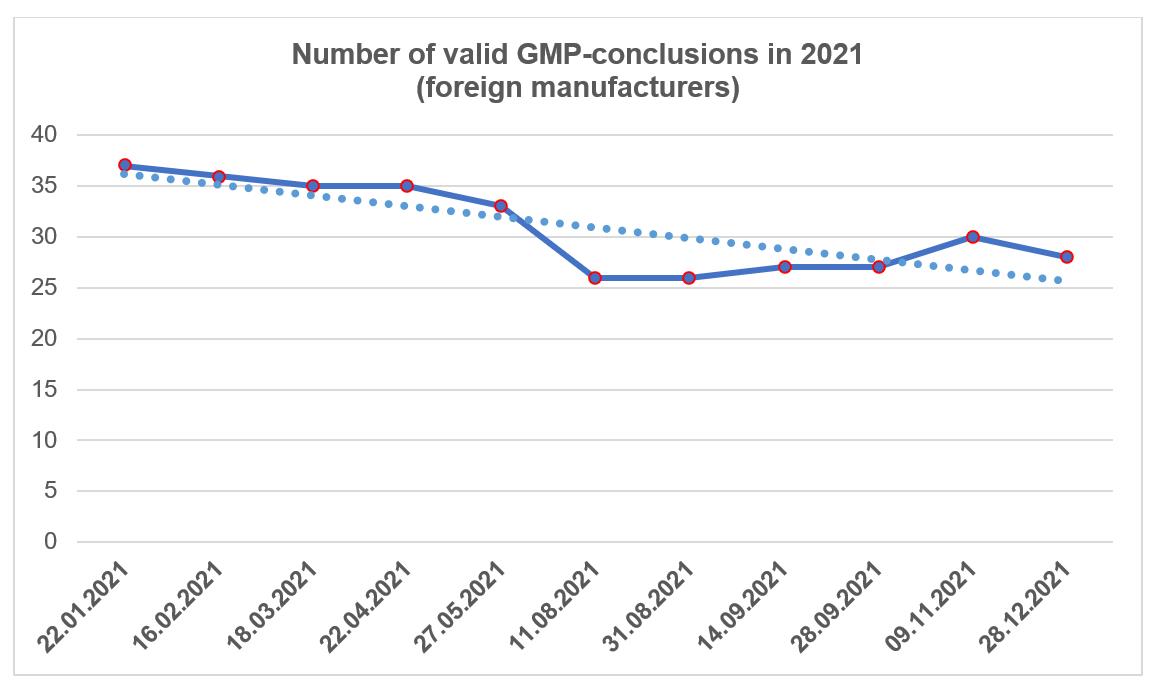
At the same time, the results of some inspections conducted in 2021 may only become known in 2022.
Commenting on the results of the inspection of foreign manufacturers, Danil Rudniaev, Deputy Director, the Head of the Inspection Body of the FSBI “VGNKI”, said during an interview with the federal specialized publication “Veterinary Medicine and Life” that in 2021, 13 out of 20 enterprises that were checked by inspectors were found to be non-compliant with the GMP requirements [4].
To date, an analysis of the results of inspections of foreign manufacturers shows the following trends:
- manufacturing sites located in Spain continue to demonstrate the best results – for 2017-2021 they received 9 GMP-conclusions;
- manufacturing sites located in the USA, Italy and Germany show the worst results – in 2017-2021 they received a total of 28 refusals to issue conclusions;
- the number of refusals to issue conclusions to sites located in France is still high, but in 2017-2021 they have already received 7 GMP-conclusions.
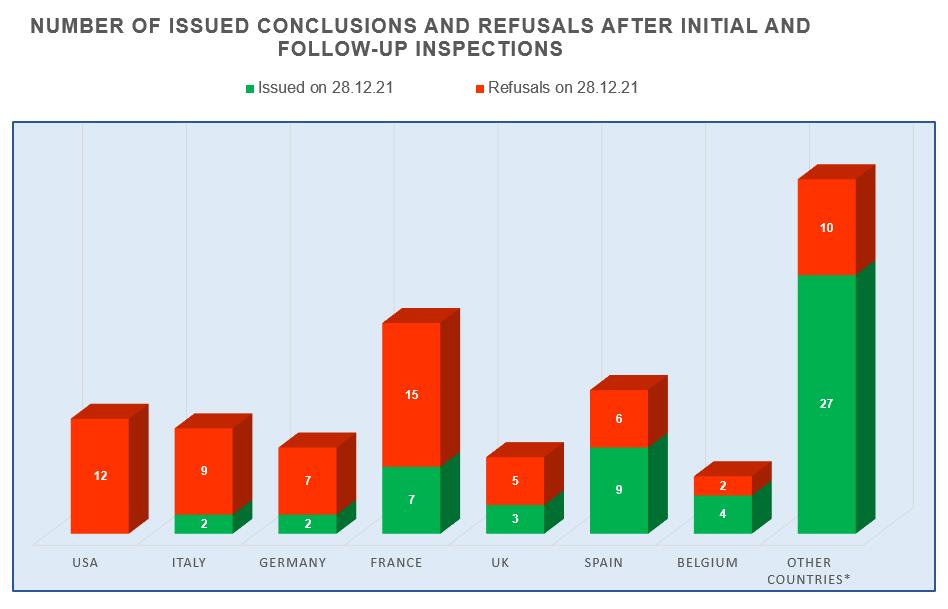
The largest number of inspections (22), based on the results of which, in 2017-2021, decisions were made to issue (refuse to issue) a GMP-conclusion, was conducted at manufacturing sites located in France. The Russian market is important for French manufacturers of veterinary medicinal products.
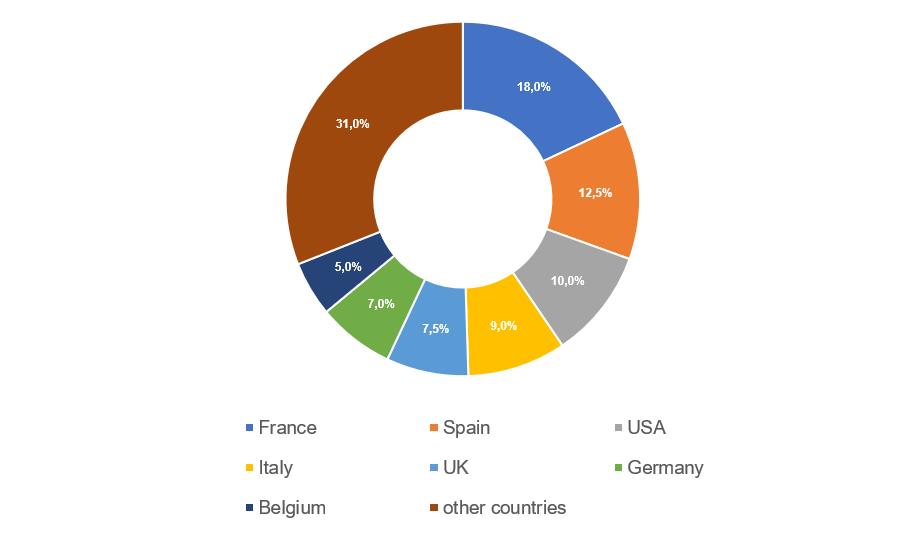
In 2021, French manufacturers underwent only 2 inspections – due to the current unfavorable situation associated with the spread of the new coronavirus infection, there are currently some restrictions on entry to France.
It should be noted that Marie-Agnès Amos, Deputy Adviser for Agriculture, Assistant for Veterinary, Sanitary, Phytosanitary Issues for Russia, Kazakhstan, Belarus, Armenia, Uzbekistan, Kyrgyzstan of the Economic Service of the French Embassy in Russia, took part in the panel discussion at the last All Russia GMP Conference [5].
Inspection in 2022
In accordance with the inspection schedule published on the FSBI “VGNKI” website 28.12.2021, 44 inspections of manufacturers of veterinary medicinal products have already been planned for 2022, which sites located in Australia, Belgium, Bulgaria, Brazil, Great Britain, Vietnam, Germany, Spain, Italy, China, the Netherlands, Slovakia, the USA, Finland, France, Croatia and Czech Republic [6].
Some of these inspections were originally planned for 2020-2021, but then they were postponed to new dates. Most of the inspections should take place in France, Spain, the USA and China.
Events and activities
In the second half of last year, many different activities were held in Russia that could be useful for manufacturers to prepare for inspection. Many exciting events and activities are also expected in 2022.
In 2021, the list of educational programs based on the Scientific and Methodological Base Center of the FSBI “VGNKI” was expanded to 80, including 7 new programs for manufacturers of medicinal products for animals [7].
In particular, in July 2021, an online seminar “Transition to the EAEU GMP Rules” was held, at which the participants of the training considered and analyzed changes in the field of requirements for pharmaceutical manufacture, personnel, premises, equipment and documentation [8].
For 2022, more than 100 training activities have been prepared, including programs in the form of individual training. The annual training schedule can be found on the website of the FSBI “VGNKI” [9].
In September 2021, the XVII Forum of business entities in the field of zoobusiness was held at the Chamber of Commerce and Industry of the Russian Federation. Polina Smyshlyaeva, Deputy Director of the Veterinary Department of the Ministry of Agriculture of the Russian Federation, presented at the Forum a complete overview of the new legislation in the field of veterinary medicine, veterinary medicinal products circulation, registration of feed additives and focused on the acts that are currently being developed [10].
“Next year we will have to settle issues of Good Distribution Practice (GDP). I think that at the beginning of next year we will start developing this document,” noted Polina Smyshlyaeva [11].
In September 2021, the VI All Russia GMP Conference with international participation, organized by the Ministry of Industry and Trade of Russia together with the FSI “SID & GP”, was held in St. Petersburg [12].
Within the framework of the Conference, a panel discussion “Inspection of manufacturers of medicines for veterinary use. Regulation and statistics. The view of the state and business” and a round table “Topical issues of inspecting manufacturers of medicinal products for veterinary use for compliance with the GMP Rules requirements” were held. They were organized by the FSBI “VGNKI” and the Association of Veterinary Pharmaceutical Manufacturers “AVFARM” [13].
More detailed information about the activities for manufacturers of veterinary medicinal products that took place within the framework of the GMP Conference can be found here: https://pharmprom.net/what-veterinary-medicines-manufacturers-ask-about/
In September 2021, the online conference “Correspondence school of the GMP/GDP auditor” was held. This annual project of the “VIALEK” Group of Companies is an important event for internal auditors of the quality system of pharmaceutical companies, managers and employees of the Quality Assurance Department, specialists in the field of Good Practice (GxP).

The calendar of upcoming activities is presented on the website of the “VIALEK” Group of Companies [14].
Eleventh Pharmaceutical Quality Week “Quality assurance of medicinal products” should take place in June 2022 in Armenia [15].
In September 2021, the SCM Pharm Community of Managers and Specialists in Logistics and Quality released the GDP Review 2 practical collected articles. Among the materials presented in the collection, one can note the article “GDP as a tool for improving the quality of operational activities” (Semyon Zhirnov, Logistics Director of GC VIC and Maria Maslennikova, Quality Manager of GC VIC) and the article “Validation of the temperature monitoring system for the transportation of medicinal products in accordance with the GxP requirements. Ensuring data integrity” (Alexander Belinsky, GxP-expert, currently – the Head of the Validation Group of the “Apicenna” company) [16].
The collection was presented to the participants of the III International Conference “Medicinal products logistics”, held in Moscow in October 2021 [17].
In October 2021, within the framework of the XXIII All Russia agro-industrial exhibition “Golden Autumn – 2021”, the FSBI “VGNKI” together with Rosselkhoznadzor held a Conference “Food quality control – the key to a healthy society” [18].
Danil Rudniaev spoke in his speech about how sites of the manufacturers of medicinal products for veterinary use are being inspected for compliance with the GMP Rules requirements during the period of restrictions associated with the pandemic of the novel coronavirus infection. In 2020, 10 out of 16 foreign manufacturing facilities were inspected online on request, and in 9 months of 2021 – 10 out of 18. In total, over two years, Rosselkhoznadzor received 44 applications from foreign companies for remote inspections, 20 of them have already been conducted, and 5 will take place before the end of the year [19].
In November 2021, with the support of the Ministry of Industry and Trade of Russia, the Annual ISPE EAEU Conference was held in Moscow [20].
The “Regulatory table” began with a review of GM(D)P regulations in the EAEU. Mkrtich Shakaryan, the Head of the Department for Inspection of GxP of the SCDMTE of the Ministry of Health after Academician Gabrielyan (Republic of Armenia), in his speech focused on the process and programs of training pharmaceutical inspectors. In particular, he noted that cooperation between regulators and professional organizations such as ISPE is mutually effective. Thus, ISPE guidelines are used by pharmaceutical companies and pharmaceutical inspectorates, a number of standards are used for the EAEU countries (standards for the use of purified water have been adopted). The ISPE educational platform is useful for pharmaceutical inspectors. “We, as representatives of the inspectorate, are interested in improving the qualifications of our employees in such areas as the validation of technological processes, analytical methods, cleaning and computerized systems,” said Mkrtich Shakaryan [21].
At the session “Harmonization of requirements for the manufacture of medicinal products” Evgenia Oleinikova, Advisor to the General Director for Quality Assurance of “SKOPINFARM” made a report on the practical implementation of GDP requirements in the context of GMP Rules. The EAEU GMP Rules contain some clauses that apply both to the distribution of medicinal products and, in some cases, to the distribution companies themselves. For example, Chapter 1 (“Pharmaceutical Quality System”) of the EAEU GMP Rules states that “The distribution of the products minimizes any risk to their quality and takes account of Good Distribution Practice”, and Chapter 5 (“Production”) says that “For the approval and maintenance of suppliers of active substances and excipients, audits should be carried out at the manufacturers and distributors of active substances. The holder of the manufacturing authorization shall verify such compliance either by himself or through an entity acting on his behalf under a contract,” emphasized the representative of “SKOPINFARM” [22].
Jean-François Duliere, a member of the EMA working group, ex-President of ISPE France Affiliate, spoke about the planned changes in Annex 1 of the EU GMP. As the speaker recalled, this Annex was first adopted in 1989 and has been revised several times, but the first proposal for a complete revision took place only in 2012. By November of last year, the EMA had received 2,000 comments on the twelfth version of the draft revised document. Many changes were made to the draft of the new version of the application [23].
In the fourth quarter of 2021, the Eurasian Academy of Good Practices held a series of free expert advisory webinars for substance manufacturers. The moderator of the past webinars was Victoria Gortinskaya, Deputy General Director for Educational Activities of the “PHARMSTRATEGY” company [24].
In November, as part of the Pharmtech & Ingredients 2021 exhibition of equipment, raw materials and technologies for pharmaceutical production, the Academy presented a simulation virtual pharmaceutical complex “Virtual Plant 2.0”, which had already been presented at the GMP Conference. The scenario of the characters’ actions inside the virtual simulation consists of several parts, each of which most realistically reflects the process of passing the pharmaceutical inspection of medicinal products production for compliance with the GMP requirements [25].
A plan of educational activities for 2022 has been published on the Academy’s website. In particular, a refresher course “Training of auditors of medicinal products production” is planned for February 2022 [26].
Planned changes within the EAEU
Currently in the Russian Federation in relation to medicinal products for veterinary use, the “Rules for organization and conduct of inspection of manufacturers of medicinal products for compliance with the GMP Rules requirements, as well as the issuance conclusions on the compliance of the manufacturer of medicinal products with the specified requirements”, approved by the Decree of the Government of the Russian Federation No. 1314 of December 03, 2015 “On determining the compliance of manufacturers of medicinal products with the GMP Rules requirements” are in force [27].
In October 2021, the EEC Legal Portal published the Order of the EEC Board No. 170 “On the draft Resolution of the Council of the Eurasian Economic Commission “On the Rules for the regulation of the circulation of veterinary medicinal products in the customs territory of the Eurasian Economic Union” [28].
Appendix 26 to the approved draft Resolution – Rules for conducting pharmaceutical inspections. These Rules will establish a common procedure for conducting pharmaceutical inspections of the manufacture of veterinary medicinal products for compliance with the EAEU GMP Rules requirements. In particular, the Rules provide for the following:
- Inspection is carried out by pharmaceutical inspectors of an authorized body (authorized organization) in the field of circulation of veterinary medicinal products or by the veterinary pharmaceutical inspectorate of the EAEU Member State
- Where nonconformities have been identified during inspection, the inspected entity in the field of circulation of veterinary medicinal products, no later than 30 calendar days from the date of receipt of the report , shall send a response to the authorized body that organized the inspection with an attached plan of corrective and preventive actions (CAPA-plan), and a report on its implementation with materials confirming the fact of their implementation, with which the lead pharmaceutical inspector and all inspection team members that conducted the inspection should be familiar
- Within 30 calendar days from the date of receipt of the response with the CAPA-plan and the report on its implementation, the authorized body (authorized organization) or the veterinary pharmaceutical inspectorate shall assess the information contained in it and perform a repeated (control) inspection in order to confirm the elimination of previously identified inspection of nonconformities
- A repeated (control) inspection by the decision of the authorized body (authorized organization) or the veterinary pharmaceutical inspectorate responsible for conducting the initial inspection can be conducted by the method of documentary inspection of the documents submitted by the inspected entity (without visiting the site) or on-site inspection in order to eliminate inconsistencies identified on it during the initial inspection (onsite)
- The authorized body, within a period not exceeding 35 calendar days from the date of signing the inspection report, makes a decision to issue (refuse to issue) a certificate
- The certificate is issued by the authorized body no later than 10 calendar days from the date it makes a decision to issue a certificate, provided that the inspected entity in the field of circulation of veterinary medicinal products eliminates all critical and major nonconformities, as well as other nonconformities, if, when considered together, they represent major nonconformities
- Where critical nonconformities with the GMP Rules requirements are identified during the inspection, the authorized body may decide to suspend or revoke the previously issued certificate, about which, within 5 working days from the date of completion of the inspection, notifies the inspected entity in writing, as well as the authorized bodies of other Member States and EEC.
During an interview with the publication “Veterinary Medicine and Life”, when asked what awaits the field of veterinary medicinal products next year, Danil Rudniaev replied: “We are waiting for a long time for a document on the rules for regulating the circulation of medicinal products for veterinary use in the EAEU market. This draft has been discussed for a very long time, starting in 2015. We expected it to be released in 2019, but discussions on its content between the EAEU Member States are still ongoing. I would like this document to finally come out, come into force, and we began to work with it; because common rules, if they are on the market of the entire Eurasian Economic Union, will be “common rules of the game” for all Member States. We will understand that the processes taking place in the Russian Federation are fully equivalent to the processes taking place in the states of the Union, and we can fully trust them” [4].
Manufacturers of veterinary medicines are encouraged to take an active part in GM(D)P-related events and activities and prepare more thoroughly for GMP inspections.
The material presented was prepared using data relevant to 31.12.2021.
In case of new or additional data the article can be updated.
References:
- Евразийский экономический союз / Решение Совета Евразийской экономической комиссии от 03.11.2016 г. № 77 «Об утверждении Правил надлежащей производственной практики Евразийского экономического союза». URL: https://docs.eaeunion.org/docs/ru-ru/01411921/cncd_21112016_77 (дата обращения 12.2021)
- ФГБУ «ВГНКИ» / Главная / Пресс-центр / Новости / Результаты работы подведомственного Россельхознадзору ФГБУ «ВГНКИ» за 2021 год. URL: http://www.vgnki.ru/rezultaty-raboty-podvedomstvennogo-rosselhoznadzoru-fgbu-vgnki-za-2021-god.html (дата обращения 31.12.2021)
- Россельхознадзор / Регистрация и лицензирование / Инспектирование. Выдача заключения / Реестр заключений о соответствии производителя требованиям правил надлежащей производственной практики. URL: https://fsvps.ru/fsvps/regLicensing/conclusion/conclusionReestr.html (дата обращения 31.12.2021)
- Ветеринария и жизнь / Главная / Зообизнес / Эксперт рассказал, как проходят GMP-инспекции производителей ветпрепаратов. URL: https://vetandlife.ru/sobytiya/ekspert-rasskazal-kak-prohodyat-gmp-inspekcii-proizvoditelej-vetpreparatov/12.2021)
- Союз предприятий зообизнеса / Новости / Инспектирование производителей ветеринарных препаратов обсудили на Всероссийской GMP-конференции. URL: http://www.spzoo.ru/cntnt/default/n7045.html (дата обращения 31.12.2021)
- ФГБУ «ВГНКИ» / Структура / Отдел инспекции производства на соответствие требованиям надлежащей производственной практики / График проведения инспектирования иностранных производителей на соответствие требованиям надлежащей производственной практики. URL: http://www.vgnki.ru/assets/files/grafik-na-sajt-28122021.pdf (дата обращения 31.12.2021)
- ФГБУ «ВГНКИ» / Главная / Пресс-центр / Новости / Итоги деятельности Научно-методического базового центра ФГБУ «ВГНКИ» за 2021 год. URL: http://www.vgnki.ru/itogi-deyatelnosti-nauchno-metodicheskogo-bazovogo-centra-vserossijskogo-gosudarstvennogo-centra-kachestva-i-standartizacii-lekarstvennyh-sredstv-dlya-zhivotnyh-i-kormov-fgbu-vgnki.html (дата обращения 31.12.2021)
- ФГБУ «ВГНКИ» / Главная / Пресс-центр / Новости / В ФГБУ «ВГНКИ» прошел онлайн-семинар «Переход на правила надлежащей производственной практики Евразийского экономического союза». URL: http://www.vgnki.ru/v-fgbu-vgnki-proshel-onlajn-seminar-perehod-na-pravila-nadlezhashhej-proizvodstvennoj-praktiki-evrazijskogo-ekonomicheskogo-soyuza.html (дата обращения 31.12.2021)
- ФГБУ «ВГНКИ» / Главная / Пресс-центр / Новости / План обучающих мероприятий ФГБУ «ВГНКИ» на 2022 год. URL: http://www.vgnki.ru/plan-obuchayushhih-meropriyatij-fgbu-vgnki-na-2022-god.html (дата обращения 31.12.2021)
- Союз предприятий зообизнеса / Главная / В Москве состоялся XVII Форум субъектов предпринимательства в сфере зообизнеса. URL: http://www.spzoo.ru/cntnt/default/n7061.html (дата обращения 31.12.2021)
- Ветеринария и жизнь / Главная / События / Для продавцов ветпрепаратов разработают правила надлежащей дистрибьюторской практики. URL: https://vetandlife.ru/sobytiya/dlya-prodavtsov-vetpreparatov-razrabotayut-pravila-nadlezhashchey-distribyutorskoy-praktiki/ (дата обращения 31.12.2021)
- VI Всероссийская GMP-конференция с международным участием / Программа. URL: https://gosgmp.ru/vi-vserossijskaya-gmp-konferentsiya-programma-vi-vserossijskoj-konferentsii-2021/ (дата обращения 31.12.2021)
- ФГБУ «ВГНКИ» / Главная / Пресс-центр / Новости / Эксперты ФГБУ «ВГНКИ» приняли участие в VI Всероссийской GMP-конференции. URL: http://www.vgnki.ru/eksperty-fgbu-vgnki-prinyali-uchastie-v-vi-vserossijskoj-gmp-konferencii.html (дата обращения 31.12.2021)
- Виалек / Главная / Мероприятия. URL: https://www.vialek.ru/events/ (дата обращения 31.12.2021)
- Одиннадцатая фармацевтическая неделя качества «Обеспечение качества лекарственных средств». URL: https://www.pharm-quality.org/ (дата обращения 31.12.2021)
- ФармПром.РФ / Анонсы / SCM Pharm выпустило второй по счету «Сборник практических статей GDP Review». URL: https://pharmprom.ru/scm-pharm-vypustilo-vtoroj-po-schetu-sbornik-prakticheskix-statej-gdp-review/ (дата обращения 31.12.2021)
- Фармсовет.РФ / III Международная конференция: Логистика лекарственных средств / Конференция / Программа конференции. URL: https://xn--80aej0akkilk.xn--p1ai/#rec331272190 (дата обращения 31.12.2021)
- XXIII Всероссийская агропромышленная выставка «Золотая осень 2021» / Деловая программа / 05 октября / Конференция «Контроль качества пищевой продукции – залог здорового общества». URL: https://russianagroweek.ru/program/ (дата обращения 31.12.2021)
- ФГБУ «ВГНКИ» / Главная / Пресс-центр / Новости / В рамках «Золотой осени» эксперты ФГБУ «ВГНКИ» провели конференцию, посвящённую контролю безопасности пищевой продукции. URL: http://www.vgnki.ru/v-ramkah-zolotoj-oseni-eksperty-fgbu-vgnki-proveli-konferenciyu-posvyashhyonnuyu-kontrolyu-bezopasnosti-pishhevoj-produkcii.html (дата обращения 31.12.2021)
- ISPE ЕАЭС / Мероприятия / Конференция ISPE ЕАЭС – 2021 / Программа. URL: https://conference.ispe.ru/uploads/2021/11/ISPE_rus_1511.pdf (дата обращения 31.12.2021)
- ISPE ЕАЭС / Новости / Что нового в фармрегуляторике ЕАЭС и в части повышения квалификации инспекторов? URL: https://ispe.ru/news/chto-novogo-v-farmregulyatorike-eaes-i-v-chasti-povysheniya-kvalifikacii-inspektorov/ (дата обращения 31.12.2021)
- ISPE ЕАЭС / Новости / На чем сфокусироваться при соблюдении GDP в контексте правил GMP? URL: https://ispe.ru/news/na-chem-sfokusirovatsya-pri-sobljudenii-gdp-v-kontekste-pravil-gmp/ (дата обращения 31.12.2021)
- ISPE ЕАЭС / Новости / Что нового в части GMP ЕС? URL: https://ispe.ru/news/chto-novogo-v-chasti-gmp-es/ (дата обращения 31.12.2021)
- Евразийская Академия надлежащих практик / Об Академии / Новости и события / Евразийская академия надлежащих практик открывает серию бесплатных экспертно-консультационных вебинаров для производителей субстанций. URL: https://gxp-academy.org/about/news_and_events/428/ (дата обращения 31.12.2021)
- Евразийская Академия надлежащих практик / Об Академии / Новости и события / Евразийская Академия надлежащих практик – участник PharmTech 2021! URL: https://gxp-academy.org/about/news_and_events/442/ (дата обращения 31.12.2021)
- Евразийская Академия надлежащих практик / Образовательная деятельность / Курсы / «Подготовка аудиторов производства лекарственных средств». URL: https://gxp-academy.org/education/courses/kurs-pk-podgotovka-auditorov-proizvodstva-lekarstvennykh-sredstv-/ (дата обращения 31.12.2021)
- ФГБУ «ВГНКИ» / Структура / Отдел инспекции производства на соответствие требованиям надлежащей производственной практики / Нормативно-правовая документация. URL: http://www.vgnki.ru/assets/files/post-1314.pdf (дата обращения 31.12.2021)
- Евразийский экономический союз / Распоряжение Коллегии ЕЭК № 170 «О проекте решения Совета Евразийской экономической комиссии «О Правилах регулирования обращения ветеринарных лекарственных средств на таможенной территории Евразийского экономического союза». URL: https://docs.eaeunion.org/docs/ru-ru/01430349/err_22102021_170 (дата обращения 31.12.2021
