This review provides information on the inspection of manufacturers of veterinary medicines for compliance with the requirements of the Good Manufacturing Practice (GMP) which is carried out by specialists of the Federal State Budgetary Institution “The All-Russian State Center for Quality and Standardization of Veterinary Drugs and Feed” (FSBI “VGNKI”).
Inspection results
Foreign manufacturers
According to the register of conclusions, published on the Rosselkhoznadzor website 18.12.2023 [1], in the fourth quarter of this year, 5 conclusions on compliance with the GMP Rules requirements were issued. These conclusions were made for sites located in located in Greece, Israel, Italy and the USA.
Thus, in 2023, 20 conclusions were already issued to foreign manufacturers, which is 2 times more than in the previous year.
The largest number of Russian inspections, based on the results of which, in 2017-2023, decisions were made to issue/refuse to issue a GMP-conclusion were carried out at manufacturing sites located in Spain (31, which is 15%). There were also many inspections carried out at sites located in France (28, which is 14%).
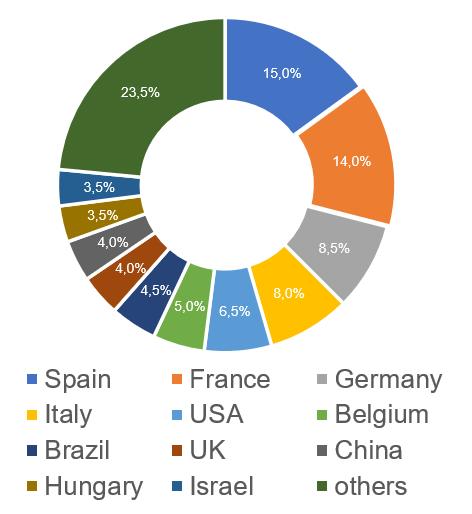
An analysis of the results of inspections in those countries where the largest number of Russian inspections were carried out shows the following trends:
- the worst results are at the manufacturing sites located in the USA, Brazil and Germany (more than 80% of refusals to issue a conclusion);
- the best results are at the manufacturing sites located in Israel and Slovenia (no refusal to issue a conclusion).
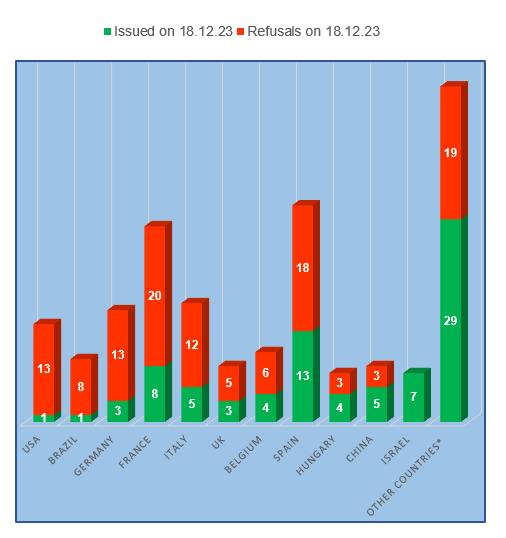
In the fourth quarter of this year, for the first time in all the years of inspection, manufacturing site from the USA was able to obtain a Russian GMP-conclusion.
According to an analysis of retail sales of veterinary medicines in Russia from January to September 2023, which was carried out by RNC Pharma [2], the USA still retains second place in the ranking of supplying countries with an indicator of 33.6%. Let us recall that in September of this year, the amendments to Federal Law No. 61-FZ of 12.04.2010 “On the Circulation of Medicines” introduced by Federal Law No. 317-FZ of 02.07.2021 [3] came into force. In accordance with these amendments, the introduction into civil circulation of each batch of a medicinal product for veterinary use imported into the Russian Federation should be carried out if only there is a conclusion on the compliance of the manufacturer of medicinal products with the requirements of the GMP Rules issued by Rosselkhoznadzor for each manufacturing site.
Inspection results for manufacturing sites located in Hungary and China have deteriorated slightly, with refusal rates increasing to 43% and 38% respectively. This situation cannot but worry manufacturers of veterinary medicines in these countries.
In October of this year, during the III International Forum “One Belt One Road” in Beijing, Russian President Vladimir Putin held an unscheduled meeting with Hungarian Prime Minister Viktor Orban. “Despite the fact that in today’s geopolitical conditions the opportunities for maintaining contacts and developing relations are very limited, nevertheless, it cannot but cause satisfaction that our relations with many European countries are maintained and developed. One of these countries is Hungary,” the President said [4]. After the opening ceremony of the forum, Russian-Chinese negotiations also took place in Beijing. Chinese President Xi Jinping said [5] that trade between Russia and China continues to grow – it has reached a historical record and is now aiming for $200 billion.
Maintaining good economic relations with these countries is in Russia’s interests. For example, this year there was news that a Hungarian company is considering Tatarstan to open a plant for the production of vials [6], and a Chinese investor will produce a pharmaceutical substance in Voskresensk [7].
The Russian market of veterinary medicines is also very interesting to Hungarian and Chinese partners, who are trying to maintain economic ties with Russia. Even though these and other countries have their own GMP Rules, manufacturers continue to study in detail the EAEU GMP Rules requirements [8] and prepare for inspection of their sites, so there remains hope for improved inspection results.
You can read about the GMP and pharmacopoeia requirements for veterinary drugs in China here (RU): https://pharmprom.ru/trebovaniya-gmp-i-farmakopei-dlya-veterinarnyx-preparatov-v-kitae/
It should be noted that the results of a number of inspections conducted in the second half of the year may become known only in the first half of 2024, so this year’s results may still change.
According to information from the FSBI “VGNKI” [9], in 2023 the Inspection Body conducted 50 inspections of foreign manufacturers of medicinal products for compliance with the GMP Rules requirements, 24 of which were carried out remotely. In addition, procedures were carried out to evaluate the submitted corrective and preventive action plans (CAPA-plans) and documents confirming the implementation of elimination of inconsistencies based on the results of inspections.
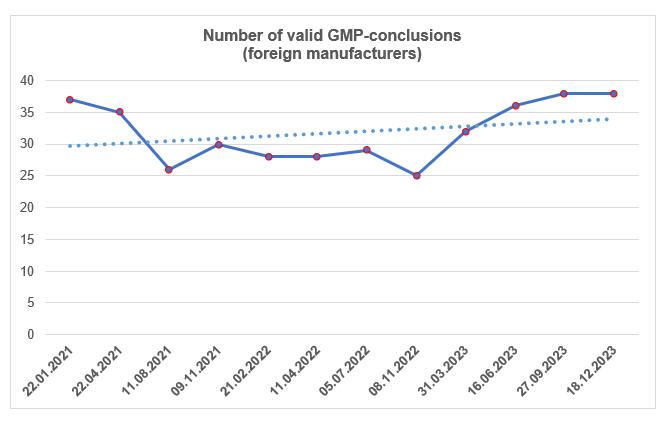
According to the register [1], by the end of the fourth quarter, the number of valid GMP-conclusions did not change. To date, foreign manufacturers have 38 valid GMP-conclusions. Over the past three years, the number of these conclusions has remained at approximately the same level, and this year it has gradually increased.
The largest number of valid conclusions now have Israeli, Spanish and Chinese sites.
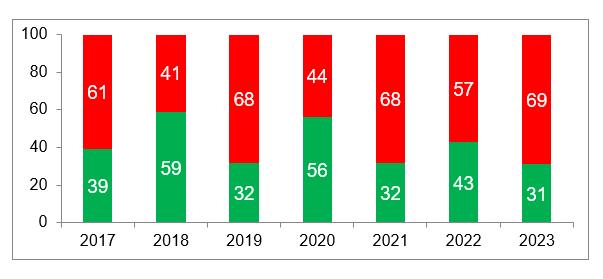
According to the data available to date, in 2023 the percentage of refusals to issue GMP-conclusions to foreign manufacturers was higher than in the previous year – 69%. At the same time, the average percentage of refusals for all years of inspection increased to 59% (for repeated inspections it is 67%, for third inspections – 70%). The average percentage of refusals for inspections related to updating previously issued GMP-conclusions is also quite high – 58%.
In accordance with the inspection schedule published on the VGNKI website 07.12.2023 [10], 12 inspections of manufacturers of veterinary medicinal products are already planned for the first half of 2024, which sites located in Argentina, Germany, India, Korea, Turkey and France.
Russian manufacturers
The latest version of the register on the Rosselkhoznadzor website [1] contains no data on the issuance of new GMP-conclusions to sites of domestic manufacturers in the fourth quarter.
According to the register, in total, 6 conclusions were issued to Russian manufacturers in 2023. To date, domestic manufacturers have 28 valid conclusions.
Let us recall that based on the results of periodic confirmation of compliance, it is possible to obtain a conclusion on compliance with the GMP Rules requirements.
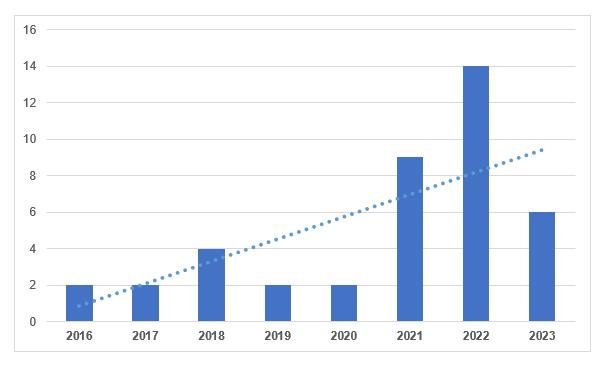
According to the Ministry of Agriculture, currently in Russia 99 Russian manufacturers of veterinary medicines meet GMP requirements (13% are government organizations, 87% are commercial). In total, 2262 veterinary medicines are registered in the Russian Federation, including 1387 domestically produced (61.3%) [11].
As part of import substitution, Russian manufacturers registered 30 veterinary vaccines and 81 pharmaceuticals for animals in 2023, the press service of Rosselkhoznadzor reported to the federal industry periodical “Veterinary Medicine and Life” [12].
Russian companies producing veterinary medicines intend to increase production capacity by 40-60% in the near future. Timur Chibilyaev, executive director of the National Veterinary Association (NVA), spoke about this at the XV International Scientific and Practical Conference “Pig Breeding-2023” [13].
Past events and activities
In the fourth quarter of this year, several events and activities took place that could be useful for manufacturers to prepare for the inspection.
On October 20, the Resolution of the EEC Council dated September 27, 2023 No. 101 “On amendments to the Decision of the Council of the Eurasian Economic Commission dated January 21, 2022 No. 1” came into force [14]. The adopted Resolution of the EEC Council guarantees mutual recognition of GMP certificates of the Eurasian Economic Union.
Recently, Rosselkhoznadzor published on its website a list of manufacturers of medicinal products for veterinary use in the Republic of Belarus that have the appropriate GMP certificate [15].
In October of this year, the V International Conference “Medicinal products logistics” was held in Moscow [16]. The community of managers and specialists in logistics and quality SCM Pharm presented all conference participants with the GDP Review 4 practical collected articles. Among the materials presented in the collection, one can note the article “On the issue of vehicles qualification” (associate professors of the Department of Industrial Pharmacy of Sechenov University Sergey Greybo, Vasily Belyaev and Natalya Nikolenko) [17]. This article deals with the qualification of vehicles of a transport company that specializes in the transportation of medicinal products. The authors of the article note that transportation is one of the most poorly studied issues of medicinal products circulation.
In accordance with the requirements of the Rules of Good Distribution Practice of medicinal products for veterinary use [18], which came into force in Russia in September 2023, during the transportation of medicines the storage conditions specified in the instructions for their use must be ensured.
In October, as part of the International specialized exhibition of feed, feed additives, veterinary medicine and equipment “KormVet-2023”, a round table was held on the topic “Prospects for the development of the veterinary industry.” During the event, speakers discussed the procedure for introducing medicines into civil circulation, the specifics of inspecting foreign manufacturers of medicinal products for veterinary use, as well as the introduction of means for the specific prevention of animal diseases as part of the import substitution strategy.
In particular, Danil Rudniaev, Deputy Director of the FSBI “VGNKI” spoke about the features of conducting inspections of foreign manufacturers of medicinal products for veterinary use [19]:
- All communications with applicants in preparation for inspection will now be carried out not by inspectors, but by the Department of International Cooperation of the FSBI “VGNKI” (when preparing documents for inspection: preparation, coordination, publication of inspection schedules; preparation and transfer of documents for inspection to the site representative; booking a hotel and preparing routes; during inspection: checking CVs and interviewing interpreters; translation support for the inspection; transfer of the inspection report to the site representative; when assessing CAPA documents: preparation and approval of an additional agreement; transfer of documents to the site representative)
- In addition to those interpreters provided by the applicant, a separate interpreter from the FSBI “VGNKI” will have to take part in the inspection, whose services will be paid for by the applicant
- If inspectors have difficulties crossing the border into EU countries (for example, attempts are made to take away their phones or laptops), the inspectors will return, the inspection will be canceled and its cost will not be returned to the applicant.

On the website of the FSBI “VGNKI” in the section “Department of production inspection for compliance with the Good Manufacturing Practice requirements” (subsection “Useful information”) the Form of a standard agreement concluded with authorized representatives of foreign manufacturers of medicinal products for veterinary use was posted [20, 21], which updated the provisions regarding the service of translation from the language of the applicant’s country or other foreign language into Russian during the inspection. The FSBI “VGNKI” reserves the right to make a decision unilaterally on the competence and approval of interpreters, who are planned to provide translation services during the inspection.
Translation issues are discussed in the article by the head of GMP-inspection.com, Alexander Podarevsky, “Interpreters at GMP inspections: difficulties and “risk analysis,” which was published in the second issue of the industry magazine “PHARMPROM” for 2023 in online format [22]. The author talks about what we should pay attention to when choosing professional interpreters with experience in supporting GMP inspections and knowledge of pharmaceutical terminology.
In November, a TASS press conference was held dedicated to summing up the results of the international competition “GxP-Profi 2023” [23].
At this press conference, Chinara Mambetalieva, the executive director of the department of technical regulation and accreditation of the EEC said: “The commission’s immediate plans include preparing an update to sections of the EAEU GMP Rules. We plan to introduce a section on organizing the Manufacture of Advanced Therapy Medicinal Products, as well as update the Manufacture of Sterile Medicinal Products.”
In December, the GхP Training Center hosted the Forum of Validation Specialists [24]. The event brought together specialists from various sectors of the pharmaceutical industry involved in validation processes. The forum has become a unique platform for exchanging experiences, discussing current issues and trends in the field of validation, as well as for finding new ideas and solutions.
In the same month, at the site of the NTFF Polysan plant in St. Petersburg, pharmaceutical industry experts gathered for a specialized workshop organized by the Eurasian Branch of ISPE (ISPE EAEU). The topic of discussion was knowledge management in the pharmaceutical production.
In particular, at this event, Svetlana Skorik, Quality Director of NTFF Polysan, spoke about knowledge management when organizing the transfer of products and technology. Based on the experience of the Polysan company, she gave a step-by-step assessment of the knowledge management system, showing how the company’s existing experience and knowledge at each time period allowed for the successful transfer of products and technologies, and also shared with the audience practical experience in overcoming technological and organizational difficulties, accompanying transfer processes [25].
Oxana Pryanichnikova, Deputy Director of the ISPE EAEU and General Director of PQE CIS expressed gratitude to the manufacturer Polysan for its openness to the innovative format and to all the speakers who provided an excellent introduction and best practices based on the ISPE Good Practice Guide: Knowledge Management in the Pharmaceutical Industry) [26].

Manufacturers are encouraged to take an active part in GM(D)P-related events and activities and prepare more thoroughly for GMP inspections.
The material presented was prepared using data relevant to 24.12.2023. In case of new or additional data the article can be updated.
Konstantin Morozov, GMP Specialist, Auditor of Pharmaceutical Enterprises
The Federal State Budgetary Institution “The All-Russian State Center for Quality and Standardization of Veterinary Drugs and Feed”, FSBI “VGNKI” is an institution authorized to conduct inspections of manufacturers of medicinal products for veterinary use manufactured outside the Russian Federation for compliance with the GMP Rules requirements
The National Veterinary Association, NVA is a non-profit organization that is a collaboration of key biopharmaceutical companies
Eurasian branch of ISPE, ISPE EAEU is a local branch of ISPE in the Eurasian Economic Union, created to provide expert support for the development of Good Practices in the pharmaceutical industry on the territory of the EAEU
REFERENCES:
- Россельхознадзор / Деятельность / Госуслуги / Выдача заключения о соответствии производителя (иностранного производителя) лекарственных средств для ветеринарного применения требованиям правил надлежащей производственной практики / Дополнительная информация для заявителя / Государственный реестр заключений о соответствии производителя (иностранного производителя) лекарственных средств для ветеринарного применения требованиям правил надлежащей производственной практики. URL: https://fsvps.gov.ru/ru/gosuslugi/zaklyuchenie-o-sootvetstvii (дата обращения 12.2023)
- RNC Pharma /Новости / RNC Pharma представляет обновление БД Аудит розничных продаж ВетЛП в России (total sell out) за сентябрь и 1-3 кв. 2023 г. URL: https://rncph.ru/news/28_11_2023 (дата обращения 24.12.2023)
- Электронный фонд правовых и нормативно-технических документов / Федеральный закон от 02.07.2021 г. № 317-ФЗ «О внесении изменений в Федеральный закон «Об обращении лекарственных средств». URL: https://docs.cntd.ru/document/607142404 (дата обращения 12.2023)
- Известия / Рубрики / Мир / Путин на встрече с Орбаном указал на развитие отношений РФ и Венгрии. URL: https://iz.ru/1590716/2023-10-17/stalo-izvestno-o-nachale-peregovorov-putina-i-orbana-v-kitae (дата обращения 24.12.2023)
- Известия / Рубрики / Экономика / Си Цзиньпин указал на приближение товарооборота КНР и России к $200 млрд. URL: https://iz.ru/1591245/2023-10-18/si-tczinpin-ukazal-na-priblizhenie-tovarooborota-knr-i-rossii-k-200-mlrd (дата обращения 24.12.2023)
- ФармПром.РФ / Новости фармацевтической отрасли / Венгерская компания рассматривает Татарстан для открытия завода о производству флаконов. URL: https://pharmprom.ru/vengerskaya-kompaniya-rassmatrivaet-tatarstan-dlya-otkrytiya-zavoda-o-proizvodstvu-flakonov/ (дата обращения 24.12.2023)
- ФармПром.РФ / Новости фармацевтической отрасли / Китайский инвестор будет производить в Воскресенске фармацевтическую субстанцию. URL: https://pharmprom.ru/kitajskij-investor-budet-proizvodit-v-voskresenske-farmacevticheskuyu-substanciyu/ (дата обращения 24.12.2023)
- Евразийский экономический союз / Решение Совета Евразийской экономической комиссии от 03.11.2016 г. № 77 «Об утверждении Правил надлежащей производственной практики Евразийского экономического союза». URL: https://docs.eaeunion.org/docs/ru-ru/01411921/cncd_21112016_77 (дата обращения 24.12.2023)
- ФГБУ «ВГНКИ» / Пресс-центр / Новости / 18 площадок иностранных производителей лекарственных средств признаны не соответствующими правилами GMP в 2023 году. URL: https://www.vgnki.ru/18-ploshhadok-inostrannyh-proizvoditelej-lekarstvennyh-sredstv-priznany-ne-sootvetstvuyushhimi-pravilami-gmp-v-2023-godu.html (дата обращения 24.12.2023)
- ФГБУ «ВГНКИ» / Структура / Отдел инспекции производства на соответствие требованиям надлежащей производственной практики / График проведения инспектирования иностранных производителей на соответствие требованиям надлежащей производственной практики. URL: https://www.vgnki.ru/assets/files/2023/grafik-na-sajt-07122023.pdf (дата обращения 24.12.2023)
- Ветеринария и жизнь / Зообизнес / Российские производители ветпрепаратов готовы выйти на 100%-е импортозамещение. URL: https://vetandlife.ru/sobytiya/rossijskie-proizvoditeli-vetpreparatov-gotovy-vyjti-na-100-e-importozameshhenie/(дата обращения 24.12.2023)
- Ветеринария и жизнь / Зообизнес / Российские производители за 2023 год зарегистрировали 30 вакцин и 81 фармпрепарат для животных. URL: https://vetandlife.ru/sobytiya/rossijskie-proizvoditeli-za-2023-god-zaregistrirovali-30-vakcin-i-81-farmpreparat-dlya-zhivotnyh/ (дата обращения 24.12.2023)
- GxP News / Новости / Российские ветпроизводители готовы увеличить мощности в полтора раза. URL: https://gxpnews.net/2023/12/rossijskie-proizvoditeli-vetpreparatov-gotovy-uvelichit-moshhnosti-v-15-raza/ (дата обращения 24.12.2023)
- Евразийский экономический союз / Решение Совета ЕЭК от 27 сентября 2023 года № 101 «О внесении изменений в Решение Совета Евразийской экономической комиссии от 21 января 2022 г. № 1». URL: https://docs.eaeunion.org/docs/ru-ru/01541473/err_10102023_101 (дата обращения 24.12.2023)
- Россельхознадзор / Главное / Россельхознадзор информирует об обращении ветеринарных препаратов белорусского производства в России. URL: https://fsvps.gov.ru/news/rosselhoznadzor-informiruet-ob-obrashhenii-veterinarnyh-preparatov-belorusskogo-proizvodstva-v-rossii/ (дата обращения 24.12.2023)
- SCM Pharm / Мероприятия / V Международная конференция Логистика лекарственных средств. URL: https://scmpharm.ru/events/5-offline-conf/#terms (дата обращения 24.12.2023)
- ФармПром.РФ / Материалы экспертов / К вопросу о квалификации машин. URL: https://pharmprom.ru/k-voprosu-o-kvalifikacii-mashin/ (дата обращения 24.12.2023)
- Официальный интернет-портал правовой информации / Приказ Министерства сельского хозяйства Российской Федерации от 29.03.2023 № 313 «Об утверждении Правил надлежащей дистрибьюторской практики лекарственных препаратов для ветеринарного применения». URL: http://publication.pravo.gov.ru/document/0001202306010055 (дата обращения 12.2023)
- ФармПром.РФ / Регуляторы фармрынка / Важные новшества связанные с инспектированием иностранных производителей ветпрепаратов. URL: https://pharmprom.ru/vazhnye-novshestva-svyazannye-s-inspektirovaniem-inostrannyx-proizvoditelej-vetpreparatov/ (дата обращения 24.12.2023)
- ФГБУ «ВГНКИ» / Структура / Отдел инспекции производства на соответствие требованиям надлежащей производственной практики / Полезная информация / Форма типового соглашения (договора), заключаемого с уполномоченными представителями иностранных производителей лекарственных средств для ветеринарного применения. URL: https://www.vgnki.ru/assets/files/2023/forma-soglasheniya-dlya-razmeshheniya-na-sajte.pdf (дата обращения 24.12.2023)
- ФармПром.РФ / Регуляторы фармрынка / Трудности перевода при инспектировании иностранных производителей ветпрепаратов. URL: https://pharmprom.ru/trudnosti-perevoda-pri-inspektirovanii-inostrannyx-proizvoditelej-vetpreparatov/ (дата обращения 24.12.2023)
- ФармПром.РФ / Анонсы / Вышел второй номер отраслевого журнала «ФАРМПРОМ» за 2023 год в формате онлайн. URL: https://pharmprom.ru/vyshel-vtoroj-nomer-otraslevogo-zhurnala-farmprom-za-2023-god-v-formate-onlajn/ (дата обращения 24.12.2023)
- ТАСС / ЕЭК намерена обновить правила производства лекарств. URL: https://tass.ru/ekonomika/19285819 (дата обращения 24.12.2023)
- ФармПром.РФ / События / Форум специалистов по валидации в учебном центре GxP. URL: https://pharmprom.ru/forum-specialistov-po-validacii-v-uchebnom-centre-gxp/ (дата обращения 24.12.2023)
- Национальный фармацевтический журнал / Новости / Экспертный воркшоп Евразийского отделения ISPE по управлению знаниями в фармпроизводстве (Knowledge Management). URL: https://npjnews.com/ispe-eaeu-mafi-eaes/ekspertnyj-vorkshop-evrazijskogo-otdeleniya-ispe-po-upravleniyu-znaniyami-v-farmproizvodstve-knowledge-management/?utm_medium=email&utm_source=Unisender&utm_campaign=311561250 (дата обращения 24.12.2023)
- ISPE / Publications / Guidance documents / Good practice guide: knowledge management in pharmaceutical industry. URL: https://ispe.org/publications/guidance-documents/good-practice-guide-knowledge-management-pharmaceutical-industry (дата обращения 24.12.2023)

