
The year 2024 is ending. The review for the fourth quarter of 2024 provides information on the inspection of manufacturers of veterinary medicines for compliance with the requirements of the Good Manufacturing Practice (GMP). These inspections are carried out by specialists of the Inspection Body of the Federal State Budgetary Institution “The All-Russian State Center for Quality and Standardization of Veterinary Drugs and Feed” (FSBI “VGNKI”).
Foreign manufacturers
According to the register of conclusions, published on the Rosselkhoznadzor website 28.12.2024 [1], in the fourth quarter of this year, 11 conclusions on compliance with the GMP Rules requirements were issued. These conclusions were made for sites located in Belgium, Brazil, Hungary, Spain, Italy, Portugal, Slovenia and the USA.
The largest number of Russian inspections, based on the results of which, in 2017-2024, decisions were made to issue/refuse to issue a GMP-conclusion were carried out at manufacturing sites located in Spain and France (a total of 75, which is 29%).
Advisor to the Director of FSBI “VGNKI” Danil Rudniaev noted the continuing reduction in the number of remote inspections (a total of 92 inspections were conducted online). According to him, conducting online inspections is a temporary measure and will not be able to completely replace the format of site inspections with specialists visiting the site of its activities in the future [2].

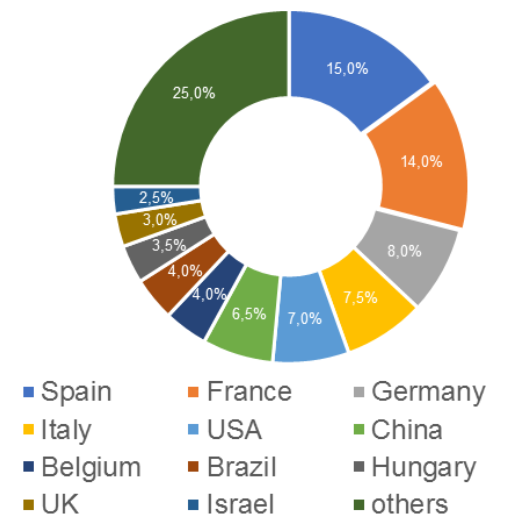
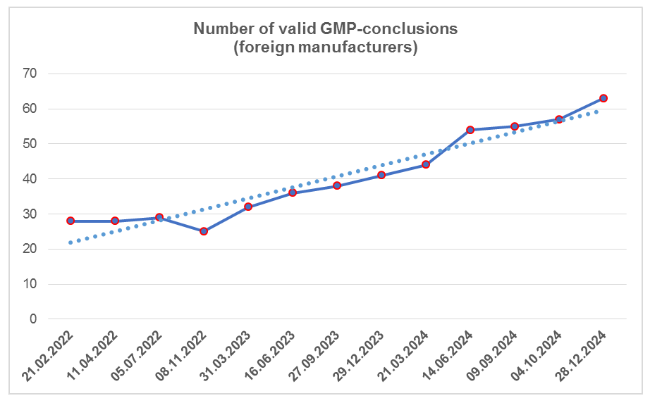
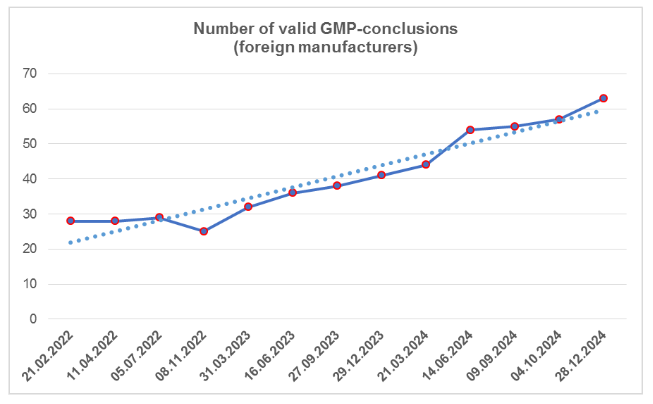
According to the register [1], by the end of the fourth quarter, the number of valid GMP-conclusions increased; despite the fact that three of them had expired (an increase has been observed over the past two years). To date, foreign manufacturers have 62 valid GMP-conclusions. The largest number of conclusions is from manufacturing sites located in Spain and China (a total of 17, which is 27%).
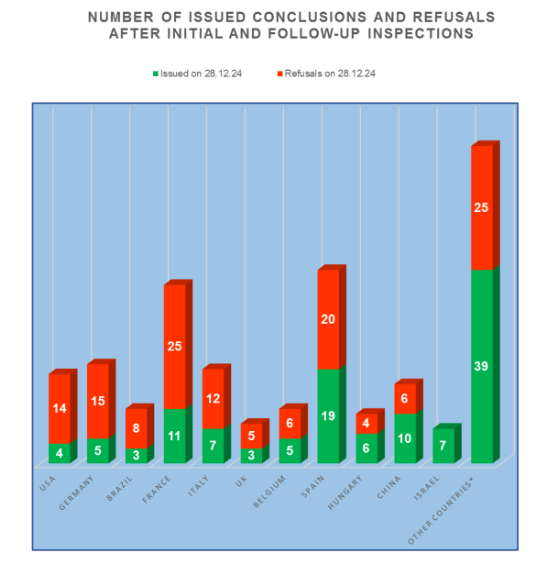
An analysis of the results of inspections in those countries where the largest number of Russian inspections were carried out shows trends similar to those of 2023:
- the worst results are at the manufacturing sites located in the USA, Germany and Brazil (more than 70% of refusals to issue a conclusion);
- the best results are at the manufacturing sites located in Israel and Slovenia (no refusal to issue a conclusion).
The largest number of valid GMP-conclusions is currently held by the manufacturing sites and CMOs of Elanco Animal Health and Zoetis, located in different countries. According to the Zooinform website, over the past few months, the Elanco company has focused its efforts on stabilizing the import of critically important veterinary medicines to Russia and ensuring that its products and manufacturing sites comply with the regulatory requirements of Russian legislation [3].
The Czech company Bioveta received a GMP-conclusion in the first half of this year. The manufacturer had been preparing for the inspection for the past year and a half. The production processes were rebuilt, the equipment was updated, and all documentation was brought into compliance. As reported by the Zooinform website, this company recently hosted a delegation from Russia [4]. Commenting on the sales volumes of veterinary medicines on the Russian retail market, Development Director of RNC Pharma Nikolay Bespalov particularly noted the success of the Slovenian company KRKA, whose products replace medicines from other manufacturers that are not available on the market [5]. Recently, this company once again confirmed the compliance of its two Slovenian sites with the requirements of the EAEU GMP Rules, approved by the Resolution of the EEC Council dated 03.11.2016 No. 77 [6]. Russia is one of five countries where KRKA has its own pharmaceutical production.
It should be noted that the results of a number of inspections conducted in the second half of the year may become known only in the first half of 2025, so this year’s results may still change.
In accordance with the inspection schedule published on the FSBI “VGNKI” website 17.12.2024 [7], 9 inspections of manufacturers of veterinary medicinal products are already planned for the first half of 2024, which sites located in Argentina, Italy, Spain, China, the Netherlands, Uruguay and France.
Russian manufacturers
According to the register on the Rosselkhoznadzor website [1], 7 GMP-conclusions were issued in the fourth quarter of this year. These conclusions were received by the manufacturing sites of MOSAGROGEN, AGROFARM, AREAL MEDICAL, FCTRBS-ARRVI, NPF VIС, T-HELPER CT and VEDA.
In total, 11 conclusions were issued to Russian manufacturers in 2024. To date, domestic manufacturers have 31 valid conclusions.
According to Ksenia Pugacheva, Head of the Department of legal regulation in the sphere of circulation of medicines, feed and feed additives for animals of the Veterinary department of the Ministry of Agriculture of Russia, 95 manufacturers currently produce veterinary products in the Russian Federation, of which 13% are state organizations and 87% are commercial organizations. Russian manufacturers are increasing production volumes, in particular vaccines. According to the results of the first ten months of this year, the volume of veterinary vaccine production has several times exceeded the 2023 figure [5].
Past events and activities
In the fourth quarter of this year, several events and activities took place that could be useful for manufacturers to prepare for the inspection.
In October, the VI International Conference “Medicinal products logistics” was held in Moscow [8]. The community of managers and specialists in logistics and quality SCM Pharm presented all Conference participants with the GDP Review 5 practical collected articles. Among the materials presented in the collection, one can note as many as three articles devoted to the topic of temperature deviations: “Temperature excursions during loading and unloading of medicinal products in a vehicle – whose area of responsibility?” [9], “The impact of temperature deviations during transportation on the quality of medicinal products” [10] and “Short-term temperature deviations during distribution of medical products” [11].
For the first time, the Conference included a separate session for manufacturers and distributors of veterinary medicines – “Main trends in the transportation and storage of veterinary products. Labeling and other challenges in 2024”. The speakers of the round table for the veterinary sector were Head of the Department for the organization of state supervision in the sphere of circulation of medicines for veterinary use of the State veterinary supervision department of Rosselkhoznadzor Yulia Kalinina, Project manager of the Pharma product group of CRPT Vilnur Shagiakhmetov and Managing director of the pharmaceutical 3PL operator NC Logistic Harold Vlasov.
You can read more about what distributors of veterinary medicine asked about here.

It should be clarified that since November 2024, the Order of Rosselkhoznadzor dated 11.09.2024 No. 1175 [12] came into force, which amends the form of checklists used by Rosselkhoznadzor and its territorial bodies in the implementation of federal state control (supervision) in the field of circulation of medicinal products for veterinary use (the Order dated 03.02.2022 No. 164). In particular, a question on the compliance of production facilities with the requirements of the EAEU GDP Rules, approved by the Resolution of the EEC Council dated 03.11.2016 No. 80 [13], has been added to the checklists.
In November, the 26th international exhibition of equipment, raw materials and technologies for pharmaceutical production Pharmtech & Ingredients 2024 [5] was held in Moscow. For the first time, a round table dedicated to the veterinary medicines market was held at the exhibition as part of the business program – “The market of medicinal products for veterinary use: its features, problems and prospects” . The event was moderated by Timur Chibilyaev, Executive director of the National Veterinary Association (NVA). Presentations were made by representatives of the EEC, the Ministry of Agriculture of the Russian Federation, Rosselkhoznadzor, FSBI “VGNKI”, CRPT, RNC Pharma, and Russian companies producing veterinary medicines.
Danil Rudniaev gave a presentation on the topic “Inspection from the inspector’s point of view. Experience and problems in organizing an inspection.” First, he presented statistical data on inspections and licensing checks of manufacturers of medicinal products for veterinary use. Then the speaker focused on the main issues that foreign and domestic manufacturers should work through when organizing inspections and checks. At the end of his presentation, the speaker provided a list of the most common inconsistencies during inspections:
- Aerosol particles in clean areas of Grades A, B, C are not monitored in order to record changes in levels of contamination and any system deterioration (manufacturers carry out monitoring in Grade A zones, but in areas of Grades B and C they do not do it or do it not with the frequency that is necessary).
- The shelf life of cleanroom clothing in a sterile condition is not established (this is especially true in cases where the clothing preparation service is outsourced).
- The integrity and closure of containers after filling are not properly monitored (in many cases, such control is not carried out at enterprises, despite the availability of available control methods).
- Validation was not carried out on three consecutive batches of products within the specified parameters (it is very difficult to prove that the number of validation batches can be less than three).
- The validation protocols for the process validation do not include the required information (e.g. review of critical process parameters).
- The maximum length of a campaign during cleaning validation is not taken into account (e.g., by the number of product batches in the campaign).

In December, the Pharmaceutical Forum of the ISPE Eurasian Affiliate was held in Minsk. At the event, representatives of leading pharmaceutical companies, representatives of regulatory authorities of the Republic of Belarus and the Russian Federation and other industry experts discussed the most actual issues related to the production of medicinal products. As Vladislav Shestakov, Director of the FSI “SID & GP”, noted in his speech, the ISPE Eurasian Affiliate plays an important role in uniting experts and promoting the harmonization of international requirements for the production of pharmaceutical products within the Eurasian Economic Union [14].
The first speaker after the official opening of the Forum was Alexander Moisak, Head of the GMP Department of the Good pharmaceutical practices department of Gosfarmnadzor of the Republic of Belarus. In particular, he presented typical non-conformities that were identified at the inspected enterprises by the pharmaceutical inspectorate of the Ministry of Health of the Republic of Belarus [15].
The Forum included sessions on “Updated Annex 1 of the EU GMP [16]: one year since entry into force”, “Shared manufacturing of medicinal products and prevention of cross-contamination risks: implementation of a risk-based approach”, “Pharma 4.0 scenario in Russia: towards operational efficiency and a culture of continuous improvement”, as well as a business interactive game focused on knowledge of the GMP Rules. Among the participants of the event were representatives of the veterinary pharmaceutical inspectorate of the State Institution “Belarusian State Veterinary Center” (SI “BSVC”) and companies producing veterinary medicines (AVZ, Materia Medica Holding, BelVitunipharm, Elanco, Ceva Sante Animale).
Dialogue with an expert

Oksana, thank you again for the invitation to the Forum in Belarus. Please tell me how difficult it is to organize such events and were there any challenges with organizing in 2024?
– It is always challenging and always very interesting. Neither I nor Vladimir Orlov, Director of the ISPE Affiliate, are professional event organizers, we have a different competence. Due to limited resources, we implement almost all tasks ourselves, as in a start-up, and new challenges arise every time.
When we planned to hold the first Conference of the ISPE Eurasian Affiliate after recognition in 2020, the period fell on lockdown in Moscow, and we had to move the event completely to an online format 2 weeks before the event. However, the event generated strong interest – more than 1.5 thousand unique connections, 10 hours of marathon sessions, the event ended with a live online tour of the modern site in Belgium.
In 2021, we implemented a hybrid format as part of a three-day event with a visit to two manufacturing sites in Kaluga, but in 2022 – in fact, a month before the event, we had to cancel the event, which was 99% ready in all respects, for reasons beyond our team’s control. This was a blow from which it was difficult for us to recover, so we decided to limit ourselves to holding chamber thematic events during the turbulent period.
Nevertheless, we saw a strong demand from the audience and decided to take a risk, not only resuming the annual event, but also holding it on the territory of another country. Our events are distinguished by the readiness of famous foreign speakers – authors of guidelines, active members of ISPE thematic groups on various topics of pharmaceutical production organization and at the same time – top managers of Big Pharma, to speak at our venue. However, now this opportunity is limited due to the policies of the companies in which the speakers work, despite the warm personal relationships that have been preserved.
What is interesting is that after the information campaign about holding the Forum in Belarus, we saw a wave of registrations only from Russia, a curious situation could have arisen, and only 1.5 months before the start of the event, manufacturers from Belarus responded to the event with interest. Unfortunately, it was not possible to change the hall to a larger format to accommodate everyone and we had to close registration a month before the start of the event. As a result, 150 participants from 60 companies from Russia and Belarus took part in the event.
Both you and Alexander Belinsky are managers in the division of a large international company, meaning you are quite busy employees at your main jobs. How do you manage to combine work in the company and ISPE EAEU?
– Alexander and I are colleagues and allies not only in our activities in PQE and ISPE, we have been implementing a technology project for over a year – developing a solution for automating qualification processes in the GxP sphere. At the moment, the Software has successfully passed pilot industrial operation at a large pharmaceutical enterprise in St. Petersburg, has been entered into the Rospatent register and is ready for commercial launch. As long as there is energy, interest, faith in our strengths and the future, the roles of corporate managers, technology entrepreneurs and public figures can be combined. Although I have a feeling that with greater delegation and support, we can implement many more ideas!
The past Forum was attended by representatives of regulatory bodies and authorized institutions of two EAEU countries. I know that, for example, a specialist from the FSBI “ARRIAH” subordinate to Rosselkhoznadzor took part in the ISPE EAEU 2021 Conference [17]. Do you think it is necessary for ISPE EAEU to develop contacts with regulators and subordinate institutions?
– There is definitely a demand from the industry, and we hope that the events of theISPE Eurasian Affiliate will become a permanent platform for such dialogue, following the example of ISPE international conferences, where regulators are always speakers on problematic issues.
You approached pharmaceutical manufacturers from Belarus with a proposal to organize visits to manufacturing sites for Forum participants. Unfortunately, this year such an idea could not be implemented. What benefit can such visits bring?
– With the right organization and attitude, the benefits of such industrial visits are hard to overestimate. This is a powerful synergy of theory and practice, a lively dialogue and search for solutions, new social connections, development of industry horizons and knowledge management on an enterprise scale.
I still cannot forget the admiring reviews of the ISPE EAEU Conference 2021 delegates about such a new experience for them. And, of course, at the first stage of planning the Forum program in Minsk, we intended to implement a project to organize a visit to one of the enterprises of Belarus. This time, such a task could not be accomplished for several reasons, but the two days of the Forum were already quite eventful.
Are you planning to hold the Forum next year, and can active pharmaceutical industry professionals suggest relevant agendas for possible coverage at future events?
Time has taught us to be cautious in forecasts, but immediately after the end of the event in Minsk, Vladimir Orlov, Alexander Belinsky and I began to form the organizational concept of the Forum of the ISPE Eurasian Affiliate 2025. We will believe that our plans and hopes will be fulfilled and, of course, there is nothing more valuable than proposals, tasks and ideas from industry specialists – they can send them to ispe@ispe.ru.
Manufacturers are encouraged to take an active part in GM(D)P-related events and activities and prepare more thoroughly for GMP inspections.
| The material presented was prepared using data relevant to 28.12.2024. In case of new or additional data the article can be updated. |
The Federal State Budgetary Institution “The All-Russian State Center for Quality and Standardization of Veterinary Drugs and Feed”, FSBI “VGNKI” is an institution authorized by the Federal Service for Veterinary and Phytosanitary Surveillance to conduct inspections of manufacturers of medicinal products for veterinary use manufactured outside the Russian Federation for compliance with the GMP Rules requirements
The Federal State Institution “State Institute of Drugs and Good Practices”, FSI “SID & GP” is an institution authorized to conduct inspections of manufacturers of medicinal products for human use manufactured outside the Russian Federation for compliance with the GMP rules requirements
The State Institution “Belarusian State Veterinary Center”, SI “BSVC” is an institution authorized by the Department of Veterinary and Food Supervision of the Ministry of Agriculture and Food of the Republic of Belarus to conduct pharmaceutical inspections of the production of veterinary medicinal products for compliance with the GMP Rules requirements
The National Veterinary Association, NVA is a non-profit organization that is a collaboration of key biopharmaceutical companies
The ISPE Eurasian Affiliate, ISPE EAEU is a local branch of ISPE in the Eurasian Economic Union, created to provide expert support for the development of Good Practices in the pharmaceutical industry on the territory of the EAEU
REFERENCES:
- Россельхознадзор / Деятельность / Госуслуги / Выдача заключения о соответствии производителя (иностранного производителя) лекарственных средств для ветеринарного применения требованиям правил надлежащей производственной практики / Дополнительная информация для заявителя / Государственный реестр заключений о соответствии производителя (иностранного производителя) лекарственных средств для ветеринарного применения требованиям правил надлежащей производственной практики. URL: https://fsvps.gov.ru/ru/gosuslugi/zaklyuchenie-o-sootvetstvii (дата обращения 28.12.2024)
- ФГБУ «ВГНКИ» / Пресс-центр / Новости / Сотрудники ФГБУ «ВГНКИ» приняли участие в круглом столе по ветпрепаратам на выставке Pharmtech & Ingredients-2024. URL: https://www.vgnki.ru/sotrudniki-fgbu-vgnki-prinyali-uchastie-v-kruglom-stole-po-vetpreparatam-na-vystavke-pharmtech-and-ingredients-2024.html (дата обращения 28.12.2024)
- Зооинформ / Фармкомпания Elanco продолжает поставки ветпрепаратов в Россию. URL: https://zooinform.ru/business/farmkompaniya-elanco-prodolzhaet-postavki-vetpreparatov-v-rossiyu/ (дата обращения 28.12.2024)
- Зооинформ / Фармкомпания Bioveta принимает гостей из России. URL: https://zooinform.ru/farmkompaniya-bioveta-prinimaet-gostej-iz-rossii/ (дата обращения 28.12.2024)
- ФармПром.РФ / Новости фармацевтической отрасли / Рынок лекарственных препаратов для ветеринарного применения: его особенности, проблемы и перспективы. URL: https://pharmprom.ru/rynok-lekarstvennyx-preparatov-dlya-veterinarnogo-primeneniya-ego-osobennosti-problemy-i-perspektivy/ (дата обращения 28.12.2024)
- Евразийский экономический союз / Решение Совета Евразийской экономической комиссии от 03.11.2016 г. № 77 «Об утверждении Правил надлежащей производственной практики Евразийского экономического союза». URL: https://docs.eaeunion.org/docs/ru-ru/01411921/cncd_21112016_77 (дата обращения 28.12.2024)
- ФГБУ «ВГНКИ» / Структура / Отдел инспекции производства на соответствие требованиям надлежащей производственной практики / График проведения инспектирования иностранных производителей на соответствие требованиям надлежащей производственной практики. URL: https://www.vgnki.ru/otdel-inspekcii-proizvodstva-na-sootvetstvie-trebovaniyam-nadlezhashhej-proizvodstvennoj-praktiki.html (дата обращения 28.12.2024)
- SCM Pharm / Мероприятия / VI Международная конференция Логистика лекарственных средств. URL: https://scmpharm.ru/events/6-offline-conf/ (дата обращения 28.12.2024)
- ФармПром.РФ / Экспертный материал / Температурные выходы при загрузках и выгрузках лекарственных средств в транспортное средство – чья зона ответственности? URL: https://pharmprom.ru/temperaturnye-vyxody-pri-zagruzkax-i-vygruzkax-lekarstvennyx-sredstv-v-transportnoe-sredstvo-chya-zona-otvetstvennosti/ (дата обращения 28.12.2024)
- ФармПром.РФ / Экспертный материал / Влияние температурных отклонений во время транспортировки на качество лекарственных средств. URL: https://pharmprom.ru/vliyanie-temperaturnyx-otklonenij-vo-vremya-transportirovki-na-kachestvo-lekarstvennyx-sredstv/ (дата обращения 28.12.2024)
- ФармПром.РФ / Экспертный материал / Кратковременные отклонения температуры при дистрибуции лекарственных средств. URL: https://pharmprom.ru/kratkovremennye-otkloneniya-temperatury-pri-distribucii-lekarstvennyx-sredstv/ (дата обращения 28.12.2024)
- Официальный интернет-портал правовой информации / Приказ Федеральной службы по ветеринарному и фитосанитарному надзору от 11.09.2024 № 1175 «О внесении изменений в приказ Федеральной службы по ветеринарному и фитосанитарному надзору от 3 февраля 2022 г. № 164 «Об утверждении форм проверочных листов (список контрольных вопросов), применяемых Федеральной службой по ветеринарному и фитосанитарному надзору и ее территориальными органами при осуществлении федерального государственного контроля (надзора) в сфере обращения лекарственных средств для ветеринарного применения». URL: http://publication.pravo.gov.ru/document/0001202411070017?index=1 (дата обращения 28.12.2024)
- Евразийский экономический союз / Решение Совета Евразийской экономической комиссии от 03.11.2016 г. № 80 «Об утверждении Правил надлежащей производственной практики Евразийского экономического союза». URL: https://docs.eaeunion.org/docs/ru-ru/01411930/cncd_21112016_80 (дата обращения 28.12.2024)
- ФБУ «ГИЛС и НП / Новости / 2024 / В Минске стартовал Форум ISPE ЕАЭС. URL: https://gilsinp.ru/news/v-minske-startoval-forum-ispe-eaes/# (дата обращения 28.12.2024)
- ФармПром.РФ / Новости фармацевтической отрасли / Белорусский фарминспекторат представил данные о типичных несоответствиях на Форуме ISPE ЕАЭС в Минске. URL: https://pharmprom.ru/belorusskij-farminspektorat-predstavil-dannye-o-tipichnyx-nesootvetstviyax-na-forume-ispe-eaes-v-minske/ (дата обращения 28.12.2024)
- The rules governing medicinal products in the European Union – Volume 4 – EU Guidelines for Good Manufacturing Practice for Medicinal Products for Human and Veterinary Use / Annex 1 – Manufacture of Sterile Medicinal Products. URL: https://health.ec.europa.eu/system/files/2022-08/20220825_gmp-an1_en_0.pdf (дата обращения 28.12.2024)
- Россельхознадзор / Региональные новости / Специалист ФГБУ «ВНИИЗЖ» принял участие в конференции «ISPE ЕАЭС – 2021» Евразийского отделения ISPE. URL: https://fsvps.gov.ru/news/specialist-fgbu-vniizzh-prinjal-uchastie-v-konferencii-ispe-eajes-2021-evrazijskogo-otdelenija-ispe/ (дата обращения 28.12.2024)

