
GMP Specialist, Auditor of Pharmaceutical Enterprises
The review for the second quarter of 2024 provides information on the inspection of manufacturers of veterinary medicines for compliance with the requirements of the Good Manufacturing Practice (GMP). These inspections are carried out by specialists of the Inspection Body of the Federal State Budgetary Institution “The All-Russian State Center for Quality and Standardization of Veterinary Drugs and Feed” (FSBI “VGNKI”).
Foreign manufacturers
According to the register of conclusions, published on the Rosselkhoznadzor website 14.06.2024 [1], in the second quarter of this year, 11 conclusions on compliance with the GMP Rules requirements were issued. These conclusions were made for sites located in Argentina, Brazil, Germany, Spain, China, the USA, France and the Czech Republic. Thus, since the beginning of the year, 18 foreign sites of veterinary medicines manufacturers have already received conclusions [2].
13 sites were refused to issue a GMP-conclusion, including after review of corrective and preventive action plans (CAPA) submitted based on the results of last year’s inspections. However, taking into account the latest data, we can conclude that in 2023 the percentage of refusals to issue GMP-conclusions to foreign manufacturers was lower than in 2021-2022.
The issuance of GMP-conclusions to manufacturing sites located in Argentina, Brazil and China is a very significant event.
The head of Rosselkhoznadzor, Sergei Dankvert, noted on the sidelines of the St. Petersburg International Economic Forum [3]: “As for friendly countries, all vaccine replacements were foreseen by us in advance. These are Southeast Asia, Brazil and Argentina.”
The number of inspections carried out at manufacturing sites in China continues to increase. In the second quarter, GMP-conclusions were issued to two Chinese sites.
In May of this year, Russian President Vladimir Putin and Chinese President Xi Jinping, after negotiations in Beijing, adopted a joint statement on deepening relations of comprehensive partnership and strategic interaction. According to the Russian President, Beijing and Moscow have accumulated a solid amount of practical cooperation; China is Russia’s main partner in the trade and economic sphere [4].
It should also be noted that there has been some improvement in the results of inspections carried out at manufacturing sites in France – in the second quarter, GMP-conclusions were issued to three French sites.
The largest number of valid conclusions now have Spanish, Chinese, French and Israeli sites.
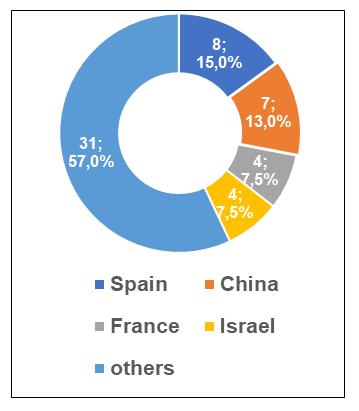
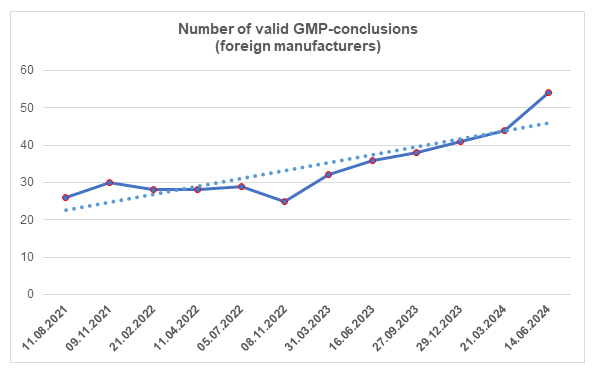
According to the register [1], by the end of the second quarter, the number of valid GMP-conclusions has increased significantly. To date, foreign manufacturers have 54 valid GMP-conclusions.
At the same time, in one of his interviews, the executive director of the AVPHARM association, Semen Zhavoronkov, noted [5]: “It is important to remember that according to the current Law [6], in order to introduce the medicine into civil circulation, all enterprises involved in its manufacturing must successfully pass Russian inspections.”
In accordance with the inspection schedule published on the VGNKI website 19.06.2024 [7], 6 inspections of manufacturers of veterinary medicinal products are planned for the third quarter of 2024, which sites located in in Belgium, Brazil, Germany, Spain, the USA and Czech Republic.
Russian manufacturers
According to the register on the Rosselkhoznadzor website [1], in the second quarter of the current year, a GMP-conclusion was issued to the NPP Farmaks manufacturing site.
To date, domestic manufacturers have 27 valid conclusions. It should be noted that Russian manufacturers produce veterinary medicines on the basis of licenses, which are issued only on condition that the enterprise complies with GMP requirements [8].
VIC Group of Companies (VIC GC) has successfully passed an audit for compliance with international requirements in the field of trade, storage and transportation of medicinal products. The company became the first and currently the only veterinary manufacturer in Russia to receive an international certificate of Good Distribution Practice (GDP). This document confirms that the logistics system of the VIC GK fully meets the international GDP requirements [9].
It should be remembered that in accordance with clause 1.8. ix of the EAEU GMP Rules, approved by the Resolution of the EEC Council dated 03.11.2016 No. 77 [10], when wholesale products are sold, it is necessary to minimize risks to its quality and take into account the Rules of Good Distribution Practice approved by the Commission [11].
In June, the Russian Government approved Resolution No. 756 of 01.06.2024 “On amendments to Resolution of the Government of the Russian Federation of March 31, 2022 No. 547” [12]. The document amends the Regulations on licensing of pharmaceutical activities. In particular, it replaces the “Rules of Good Distribution Practice of medicinal products for veterinary use” with “Rules of Good Distribution Practice within the framework of the Eurasian Economic Union” [13].
According to the conclusion of a study of the market for veterinary medicines in Russia, conducted by the National Veterinary Association (NVA), domestic manufacturers are successfully implementing an import substitution program [14].
PAST EVENTS AND ACTIVITIES
In the second quarter of this year, several events and activities took place that might be of interest to manufacturers preparing for inspection.
On April 10, the Chairman of the State Duma Committee on Agrarian Issues, Academician of the Russian Academy of Sciences Vladimir Kashin held a meeting with the participation of the Deputy Minister of Agriculture of the Russian Federation Maxim Uvaidov to consider several draft laws presented by deputies. Among other things, the meeting considered draft Law No. 555529-8 “On suspension of Part 3 of Article 52-2 of the Federal Law “On the Circulation of Medicines” [15] (draft law on suspension of the obligation to obtain a GMP certificate for veterinary medicinal products imported from abroad).
According to the Committee on Agrarian Issues, there are no grounds for suspending Part 3 of Article 52.2. of the Federal Law No. 61-FZ of 12.04.2010 “On the Circulation of Medicines” [6], and the proposed changes will not contribute to filling the domestic market with high-quality and safe veterinary medicines. Thus, the Committee on Agrarian Issues did not support the concept of the introduced draft law. The conclusion on the draft law with comments and the position of the committee was sent to the responsible committee – the State Duma Committee on Health Protection [16].
In April, the 22nd International Exhibition “Analitika Expo 2024” was held in Moscow. On April 17, within the framework of the exhibition, the “Forum of specialists on the quality and registration of medicinal products” was held, the partner of which was the GxP Training Center [17].
In her report “Review of requirements for storage of finished products”, Marina Chekrygina, GMP specialist, QA leading specialist at Firm Zdorovye LLC, reviewed the regulatory framework and compared the following topics: access, space, fit-out and cleaning of premises, storage organization, zoning, conditions and modes of storage, protection from exposure to low temperatures, mapping, location of climate control devices, protection from light, pest control, movement of medicinal products and outsourcing.
In the same month, the XIII International Pharmaceutical Forum PharmPRO 2024was held in Moscow. As part of the business program, industry trends, increasing production efficiency, ensuring quality of medicines, solving personnel problems and entering new markets with competitive products were discussed [18].
Hasmik Abrahamyan, Director of the Quality Department at GEROPHARM, took part in the session “Consistent quality of pharmaceuticals at every stage” and spoke about the digitalization of validation processes. She presented a project that resulted in the development of a software solution based on the commercially available office suite Microsoft Office. Among the efficiency factors of the developed solution, she noted the following: reducing the time for developing validation documents by more than 45%; increasing the availability of data obtained during validation tests for further processing; organization of quick access to approved validation reports.
 At the end of April, Elis Cleanroom held another free webinar on the new EU GMP Annex 1 [19] – “Reflecting on Annex 1: A comprehensive review since its implementation in 2023”.
At the end of April, Elis Cleanroom held another free webinar on the new EU GMP Annex 1 [19] – “Reflecting on Annex 1: A comprehensive review since its implementation in 2023”.
Elis Cleanroom also acted as a partner of the International Student Festival “GxP-Fest 2024”, which has been held for the fourth year by the Eurasian Academy of Good Practices with the support of the Ministry of Industry and Trade of Russia and the Eurasian Economic Commission [20].
The Eurasian Academy of Good Practices has developed a Practical course for training auditors at the manufacturing site of a pharmaceutical enterprise. The training is aimed at a comprehensive audit of manufacturing sites for compliance with GMP/GDP requirements by auditors with extensive inspection experience, a comprehensive assessment of the professional competencies of specialists based on a practice-oriented training course for internal auditors directly at the place of production activities [21].
UPCOMING EVENTS AND ACTIVITIES
On August 21-23, the IX All-Russian GMP Conference will take place in Ufa. The organizer of the conference is the Ministry of Industry and Trade of Russia together with the FSI “SID & GP”. The All-Russian GMP Conference has been held since 2016 and annually brings together leading international and Russian experts in the pharmaceutical industry, representatives of government authorities, managers and specialists of pharmaceutical production, representatives of professional associations and communities, experts in the field of quality management systems and GMP [22].
Manufacturers are encouraged to take an active part in GM(D)P-related events and activities and prepare more thoroughly for GMP inspections.
The material presented was prepared using data relevant to 22.06.2024. In case of new or additional data the article can be updated.
The Federal State Budgetary Institution “The All-Russian State Center for Quality and Standardization of Veterinary Drugs and Feed”, FSBI “VGNKI” is an institution authorized to conduct inspections of manufacturers of medicinal products for veterinary use manufactured outside the Russian Federation for compliance with the GMP Rules requirements
The National Veterinary Association, NVA is a non-profit organization that is a collaboration of key biopharmaceutical companies
The Association of Veterinary Pharmaceutical Manufacturers AVPHARM is an association representing the interests of leading international pharmaceutical companies in the Russian Federation – manufacturers of medicinal products for veterinary use (MSD Animal Health, Zoetis and Boehringer Ingelheim)
REFERENCES:
- Россельхознадзор / Деятельность / Госуслуги / Выдача заключения о соответствии производителя (иностранного производителя) лекарственных средств для ветеринарного применения требованиям правил надлежащей производственной практики / Дополнительная информация для заявителя / Государственный реестр заключений о соответствии производителя (иностранного производителя) лекарственных средств для ветеринарного применения требованиям правил надлежащей производственной практики. URL: https://fsvps.gov.ru/ru/gosuslugi/zaklyuchenie-o-sootvetstvii (дата обращения 22.06.2024)
- Ветеринария и жизнь / Законодательство / С начала года сертификаты GMP получили уже 18 зарубежных производителей ветпрепаратов. URL: https://vetandlife.ru/zakons/s-nachala-goda-sertifikaty-gmp-poluchili-uzhe-18-zarubezhnyh-kompanij/ (дата обращения 22.06.2024)
- Ветеринария и жизнь / Зообизнес / Российский рынок адаптировался к уходу производителей ветпрепаратов из недружественных стран – Сергей Данкверт. URL: https://vetandlife.ru/pet-business/rossijskij-rynok-adaptirovalsya-k-uhodu-proizvoditelej-vetpreparatov-iz-nedruzhestvennyh-stran-sergej-dankvert/ (дата обращения 22.06.2024)
- Известия / Рубрики / Мир / Путин и Си Цзиньпин приняли заявление об углублении партнерства. URL: https://iz.ru/1697243/2024-05-16/putin-i-si-tczinpin-priniali-zaiavlenie-ob-uglublenii-partnerstva?main_click (дата обращения 22.06.2024)
- GxP News / Практика / Реально ли восстановление импорта ветпрепаратов в России? URL: https://gxpnews.net/2024/05/realno-li-vosstanovlenie-importa-vetpreparatov-v-rossii/ (дата обращения 22.06.2024)
- Электронный фонд правовых и нормативно-технических документов / Федеральный закон от 12.04.2010 г. № 61-ФЗ «Об обращении лекарственных средств» (с изменениями на 1 апреля 2024 года). URL: https://docs.cntd.ru/document/902209774 (дата обращения 22.06.2024)
- ФГБУ «ВГНКИ» / Структура / Отдел инспекции производства на соответствие требованиям надлежащей производственной практики / График проведения инспектирования иностранных производителей на соответствие требованиям надлежащей производственной практики. URL: https://www.vgnki.ru/assets/2024/files/grafik-na-sajt-19062024.pdf (дата обращения 22.06.2024)
- Ветеринария и жизнь / Зообизнес / В Россельхознадзоре рассказали, как следят за качеством лекарств для животных. URL: https://vetandlife.ru/pet-business/v-rosselhoznadzore-rasskazali-kak-sledyat-za-kachestvom-lekarstv-dlya-zhivotnyh/ (дата обращения 22.06.2024)
- ВИК / Новости / Группа компаний ВИК первая в отрасли получила сертификат GDP. URL: https://vicgroup.ru/press-center/gruppa-kompaniy-vik-pervaya-v-otrasli-poluchila-sertifikat-gdp/ (дата обращения 22.06.2024)
- Евразийский экономический союз / Решение Совета Евразийской экономической комиссии от 03.11.2016 г. № 77 «Об утверждении Правил надлежащей производственной практики Евразийского экономического союза». URL: https://docs.eaeunion.org/docs/ru-ru/01411921/cncd_21112016_77 (дата обращения 22.06.2024)
- Евразийский экономический союз / Решение Совета Евразийской экономической комиссии от 03.11.2016 г. № 80 «Об утверждении Правил надлежащей производственной практики Евразийского экономического союза». URL: https://docs.eaeunion.org/docs/ru-ru/01411930/cncd_21112016_80 (дата обращения 22.06.2024)
- Официальный интернет-портал правовой информации / Постановление № 756 от 01.06.2024 «О внесении изменений в Постановление Правительства Российской Федерации от 31 марта 2022 года № 547». URL: http://publication.pravo.gov.ru/document/0001202406030030?index=2 (дата обращения 22.06.2024)
- Фармацевтический вестник / Новости / Регуляторика / Правительство внесло изменения в требования к лицензированию фармдеятельности. URL: https://pharmvestnik.ru/content/news/Pravitelstvo-vneslo-izmeneniya-v-trebovaniya-k-licenzirovaniu-farmdeyatelnosti.html (дата обращения 22.06.2024)
- НВА / Новости / Рынок ветеринарных препаратов в России – итоги 2023 года. URL: https://rosvet.org/novosti/rynok-veterinarnyh-preparatov-v-rossii-itogi-2023-goda/ (дата обращения 22.06.2024)
- СОЗД / Объекты законотворчества / Законопроект № 555529-8 «О приостановлении действия части 3 статьи 52-2 Федерального закона «Об обращении лекарственных средств». URL: https://sozd.duma.gov.ru/bill/555529-8 (дата обращения 22.06.2024)
- AgroXXI.ru / Ветеринария | Животноводство / Справляются ли производители российских ветеринарных препаратов с импортозамещением. URL: https://www.agroxxi.ru/zhivotnovodstvo/veterinarija/spravljayutsja-li-proizvoditeli-rossiiskih-veterinarnyh-preparatov-s-importozamescheniem.html (дата обращения 22.06.2024)
- Аналитика Экспо / Новости / Чем запомнился второй день выставки «Аналитика Экспо 2024». URL: https://analitikaexpo.com/ru/about/news/2024/april/17/analitika-ehkspo-2024-itogi-vtorogo-dnya/ (дата обращения 22.06.2024)
- PharmPRO / XIII фармацевтический форум PharmPRO. URL: https://events.pharmpro.pro/forum-2024 (дата обращения 22.06.2024)
- EudraLex – Volume 4 – Good Manufacturing Practice (GMP) guidelines / Annex 1 – Manufacture of Sterile Medicinal Products. URL: https://health.ec.europa.eu/document/download/e05af55b-38e9-42bf-8495-194bbf0b9262_en?filename=20220825_gmp-an1_en_0.pdf (дата обращения 22.06.2024)
- Евразийская Академия надлежащих практик / Новости / Elis Cleanroom на площадке «GxP-Феста 2024» проведет мастер-класс «Как правильно одеваться в чистых помещениях». URL: https://gxp-academy.org/news/elis-cleanroom-na-ploshchadke-gxp-festa-2024-provedet-master-klass-kak-pravilno-odevatsya-v-chistykh/ (дата обращения 22.06.2024)
- Евразийская Академия надлежащих практик / Новости / Эксперты Евразийской Академии разработали программу учебных аудитов для сотрудников фармпредприятий. URL: https://gxp-academy.org/news/preventivnaya-filosofiya-kachestva/ (дата обращения 22.06.2024)
- VIII Всероссийская GMP-конференция. URL: http://gosgmp.ru/ (дата обращения 22.06.2024)

