
There are only two months left before the amendments to Federal Law No. 61-FZ of 12.04.2010 “On the Circulation of Medicines” introduced by Federal Law No. 317-FZ of 02.07.2021 [1] come into force. From September 1, 2023, the introduction into civil circulation of each medicinal product for veterinary use imported into the Russian Federation will be carried out if only there is a conclusion on the compliance of the manufacturer of medicinal products with the requirements of the Good Manufacturing Practice (GMP) Rules issued by Rosselkhoznadzor for each manufacturing site. This review provides information on the inspection of manufacturers of veterinary medicines for compliance with the GMP Rules requirements which is carried out by specialists of the Federal State Budgetary Institution “The All-Russian State Center for Quality and Standardization of Veterinary Drugs and Feed” (FSBI “VGNKI”).
Inspection results
Foreign manufacturers
According to the register of conclusions, published on the Rosselkhoznadzor website 16.06.2023 [2], in the second quarter of this year, 5 conclusions on compliance with the GMP Rules requirements were issued. These conclusions were made for sites located in Hungary, Israel, Spain, and Turkey.
The leading country has changed, where the most Russian inspections were carried out, based on the results of which, over 6 years of inspection, decisions were made to issue/refuse to issue a GMP-conclusion (28, which is 16%) – now it’s Spain.
In one of his speeches, the general director of NVC Agrovetzaschita S-P Sergey Engashev noted that veterinary medicines from 37 countries are registered in Russia. The leader in the import of medicinal products for animals is Spain, the companies of this country supply 151 medicines to the Russian market [3].
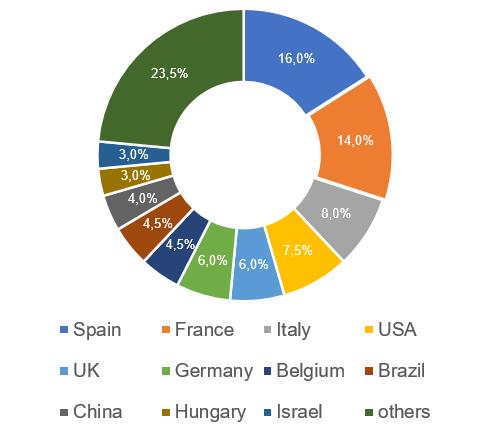
An analysis of the results of inspections in those countries where the largest number of Russian inspections were carried out shows similar trends as in the second half of last year:
- the worst results are at the manufacturing sites located in the USA, Brazil and Germany;
- the best results are at the manufacturing sites located in Israel, as well as in Hungary and China.
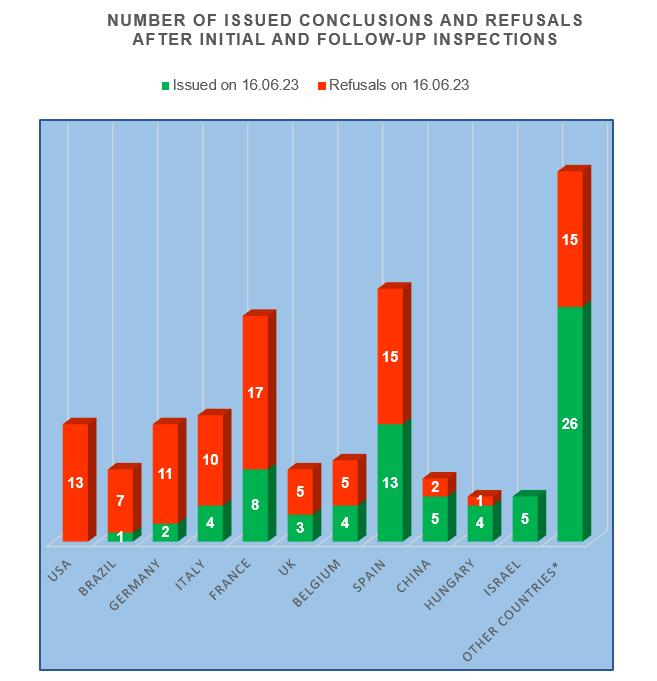
Decisions to issue/refuse to issue a GMP-conclusion to sites from the USA have not been made for quite a long time.
As for sites from Brazil, at the beginning of June this year, the FSBI “VGNKI” held a videoconference with representatives of the Brazilian National Union of the Industry for Animal Health Products (Sindan). During the conference, Yana Baginskaya, the Head of the Inspection Department of the FSBI “VGNKI”, explained the sequence of actions in preparing for the inspection, the method of submitting the application and the list of documents attached to it, the requirements for production, and also drew attention to the identification of inconsistencies in the quality of medicines for veterinary use [4].
Based on the results of inspections of sites from Israel, there was not a single refusal to issue a conclusion.
To date, the largest number of valid conclusions (13) have French, as well as Spanish and Chinese sites. Speaking at the round table “Opening the doors to our colleagues from China” within the framework of the exhibition “MVC: Cereals-Mixed Feed-Veterinary-2023” held in June, the executive director of the National Veterinary Association (NVA) Timur Chibilyaev called on Chinese partners to expand the supply of auxiliary components and technologies for the development of the production of veterinary medicines, and also noted the demand for import and localization in Russia of the production of medical and veterinary medicines by Chinese companies [5].
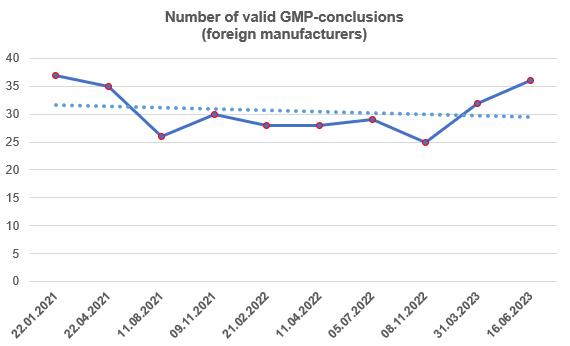
By the end of the second quarter, the number of valid GMP-conclusions slightly increased and reached approximately the same level as at the beginning of 2021.
According to Rosselkhoznadzor, to date, foreign manufacturers have 36 valid GMP-conclusions [6].
It should be borne in mind that the production and quality control process of some medicines can be shared among several manufacturing sites that perform separate stages.
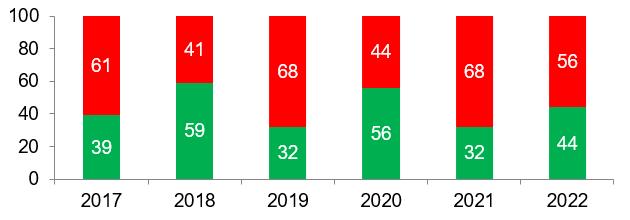
According to the data available to date, in 2022 the percentage of refusals to issue GMP-conclusions to foreign manufacturers was slightly lower than in the previous year, while the average percentage of refusals over 6 years of inspection is 57% (for repeated inspections – 65%, for third inspections – 83%). This situation cannot but worry foreign manufacturers of veterinary medicines.
The commentary of the executive director of the AVPHARM association, Semen Zhavoronkov, published in May on the ZooMedVet portal, contains the following statistics: “Since 2017, 83 inspections have already been carried out at the manufacturing facilities of AVPHARM companies, including 28 in online mode, as a result, 20 certificates have been received (only 8 of them are active this year). Efficiency is <25%” [7].
In April of this year, the information portal of the “Izvestia” newspaper wrote [8] that the deputies of the LDPR faction in the State Duma sent an appeal to the Deputy Prime Minister of the Russian Federation Viktoria Abramchenko with a request to postpone for two years the entry into force of amendments to Federal Law No. 61-FZ of 12.04.2010 “On the Circulation of Medicines” [1], which relate to the requirement for a conclusion on the compliance of the manufacturing site of a foreign manufacturer of medicinal product for veterinary use with the requirements of the EAEU GMP Rules [9].
In accordance with the inspection schedule published on the VGNKI website 30.06.2023 [10], 11 inspections of manufacturers of veterinary medicinal products are planned for the third half of 2023, which sites located in Germany, India, Italy, China, the Netherlands, New Zealand, the USA and France.
Russian manufacturers
According to the NVA, this year’s goal is to reduce the share of imports in chemical pharmaceuticals and immunobiology. And the potential of Russian manufacturers, based on market development trends, is significant: the production of vaccines since 2018 has increased by 2.3 times, hormones – by 2 times, and all categories of veterinary products – by 70% [11].
According to the register on the Rosselkhoznadzor website [2], in the second quarter of the current year, a GMP conclusion was issued to the AlexAnn manufacturing site.
To date, domestic manufacturers have 29 valid conclusions. At the same time, according to the Director of the Department of Veterinary Medicine of the Ministry of Agriculture of Russia Maria Novikova, announced during the exhibition “MVC: Cereals-Mixed Feed-Veterinary-2023”, 100 Russian manufacturers of veterinary medicines comply with GMP requirements [12].
In May of this year, the new production site of the Vetbiohim company, located in the special economic zone Technopolis – Moscow, received the Turkish GMP certificate. The certificate opens new opportunities for the company to develop export potential and establish trade relations with representatives of friendly countries [13].
Past events and activities
In the second quarter of this year, several events took place that could be useful for manufacturers to prepare for the inspection.
In April, the XXXI Moscow International Veterinary Congress MVC 2023 was held, during which the section “Business and government” was organized. Within the framework of the congress, in the section “Business and Government” of the business program of the event, Deputy Director of the FSBI “VGNKI” Vasilina Gritsyuk presented a report. The speaker spoke about the introduction of medicinal products for veterinary use into civil circulation, presented a complete list of documents regulating this procedure and explained the procedure for issuing test reports [14].
In the same week of April, Moscow hosted the 22nd Analitika Expo exhibition, the largest international exhibition of laboratory equipment and chemical reagents in Russia and Eastern Europe and the main meeting place for specialists in the field of analytical chemistry.
Within the framework of the exhibition, a “Forum for specialists in quality control of medicinal products” was organized, the partner of which was the GxP Training Center. The forum presented the following topics: “Discussion of the EAEU Pharmacopoeia”, “How to arrange the purchase of a new piece of equipment – from an idea to a working device”, “Audit of quality control laboratories”, “Incoming control policy in pharmaceutical production” [15].

In April, the XII International Pharmaceutical Forum PharmPRO 2023 was held in Moscow.
As part of the forum, at a special session “How to ensure stable production of high-quality pharmaceutical products in the face of constant changes?” such key issues as “Working with suppliers of substances in the preparation of the registration dossier”, “Quality specifications and requirements for suppliers in terms of value for the stable production of medicines”, etc. were considered [16].

In May, on the website of the Ministry of Agriculture of Russia, a section “Information on the state service for attestation of a person authorized by the holder or owner of the marketing authorization for a medicinal product for veterinary use” appeared, which discloses the details of the specified state service [17].
In accordance with the Order of the Ministry of Agriculture of Russia dated 10.11.2022 No. 795 [18], the test tasks will include fifty questions concerning the requirements of the GMP Rules, as well as the requirements for the registration dossier for a medicinal product for veterinary use.
The document “Attestation of a person authorized by the holder or owner of the MA of the VMP” presented on the site provides a list of regulatory legal acts, the knowledge of which is necessary to complete the test tasks and a list of topics of questions included in the test tasks [19].
There is currently no information from the Department of Veterinary Medicine about the possibility of early attestation.
In the second quarter of this year, a series of webinars were again dedicated to the new Annex 1 to EU GMP [20], which will be implemented in the European Union as early as next month.
For example, the PHARMSTRATEGY company held several open expert and consulting webinars on new requirements for the manufacture of sterile medicinal products in the light of the revision of Annex 1 to EU GMP, including an overview of regulatory innovations and the complexity of their implementation, new approaches to aseptic process validation and others.
In May, a free webinar “Sterilizing filtration validation: modern approaches, regulatory requirements and the main difficulties of their implementation” was held on the platform of the Eurasian Academy of Good Practices. The webinar participants were able to get up-to-date information about the approaches used, get acquainted with current trends and innovations in this area, hear expert opinions on updates in the regulatory requirements for the validation of sterilizing filtration processes in accordance with the new version of Annex 1 to EU GMP [21].
Sterilizing filtration issues are often considered during Russian inspections. For example, last year the following nonconformity was included in the “List of the most common nonconformities when inspecting manufacturers of medicinal products for veterinary use” – the integrity of sterilizing filters is not confirmed at appropriate time intervals [22].
It was possible again to take part in some international events remotely.
In April-May, free video-meetings “GMP/GDP Q&A” were held with Aleksandr Aleksandrov, an international expert, trainer-consultant, auditor of the VIALEK group of companies. During these meetings, the participants had the opportunity to ask their questions, discuss them with colleagues in a chat, or listen to the expert’s answers to questions from other participants.
In April-June, the French company Elis held a free webinars “Annex 1 Goggles” (organized jointly with Univet company) and “How Can You Responsibly Handle End-of-Life Cleanroom Garments?”, “Key garment considerations for controlled environments” (organized jointly with Alsico company).
Upcoming events and activities
In May, registration was opened for the VIII All Russian GMP Conference, which will be held in Ekaterinburg on September 26-28. The organizer of the conference is the Ministry of Industry and Trade of Russia together with the FSI “SID & GP” [23]. Representatives of Russian and foreign regulatory authorities, manufacturing sites, leading industry experts are invited to participate. During the three days of the conference, a number of thematic sessions and panel discussions will take place, as well as traditional master classes on Good Manufacturing Practices and GMP-inspections.
There is currently no information on holding round tables and thematic sessions in the veterinary field within the framework of the conference.
In September-October, on the platform of the Eurasian Academy of Good Practices, the next advanced training course “Training auditors of the production of medicinal products” will be held (correspondence form of education using distance learning technologies). Upon completion of training, participants are issued a certificate of professional development of the established sample [24].
On October 20, Moscow will host the V International Conference “Medicinal products logistics”. Registration for the event will open on August 1st. In particular, the conference will host the SCM Pharm 2023 Award Ceremony. The SCM Pharm Community of Managers and Specialists in Logistics and Quality is preparing the publication of the GDP Review 4 practical collected articles [25].
Manufacturers are encouraged to take an active part in GM(D)P-related events and activities and prepare more thoroughly for GMP inspections.
The material presented was prepared using data relevant to 04.07.2023. In case of new or additional data the article can be updated.
The Federal State Budgetary Institution “The All-Russian State Center for Quality and Standardization of Veterinary Drugs and Feed”, FSBI “VGNKI” is an institution authorized to conduct inspections of manufacturers of medicinal products for veterinary use manufactured outside the Russian Federation for compliance with the GMP rules requirements
The National Veterinary Association, NVA is a non-profit organization that is a collaboration of key biopharmaceutical companies
The National Union of the Industry for Animal Health Products – Sindicato Nacional da Indústria de Produtos para Saúde Animal, Sindan. Founded in 1966 in Brazil, the union unites more than 90 companies, which accounts for about 90% of the Brazilian market for veterinary medicines
The Association of Veterinary Pharmaceutical Manufacturers AVPHARM is an association representing the interests of leading international pharmaceutical companies in the Russian Federation – manufacturers of medicinal products for veterinary use (MSD Animal Health, Elanco, Zoetis and Boehringer Ingelheim)
REFERENCES:
- Электронный фонд правовых и нормативно-технических документов / Федеральный закон от 02.07.2021 г. № 317-ФЗ «О внесении изменений в Федеральный закон «Об обращении лекарственных средств». URL: https://docs.cntd.ru/document/607142404 (дата обращения 07.2023)
- Россельхознадзор / Деятельность / Госуслуги / Выдача заключения о соответствии производителя (иностранного производителя) лекарственных средств для ветеринарного применения требованиям правил надлежащей производственной практики / Дополнительная информация для заявителя / Государственный реестр заключений о соответствии производителя (иностранного производителя) лекарственных средств для ветеринарного применения требованиям правил надлежащей производственной практики. URL: https://fsvps.gov.ru/ru/gosuslugi/zaklyuchenie-o-sootvetstvii (дата обращения 04.07.2023)
- Ветеринария и жизнь / Зообизнес / Производители призывают продлить ускоренную регистрацию российских ветпрепаратов. URL: https://vetandlife.ru/sobytiya/proizvoditeli-prizyvajut-prodlit-uskorennuju-registraciju-rossijskih-vetpreparatov/ (дата обращения 04.07.2023)
- ФГБУ «ВГНКИ» / Пресс-центр / Новости / Сотрудники ФГБУ «ВГНКИ» приняли участие в видеоконференции с представителями бразильской ассоциации Sindan. URL: https://www.vgnki.ru/sotrudniki-fgbu-vgnki-prinyali-uchastie-v-videokonferencii-s-predstavitelyami-brazilskoj-associacii-sindan.html (дата обращения 04.07.2023)
- Зооинформ / Наращивание импорта ветпрепаратов из Китая прокомментировали в АВФАРМ. URL: https://zooinform.ru/vete/narashhivanie-importa-vetpreparatov-iz-kitaya-prokommentirovali-v-avfarm/ (дата обращения 04.07.2023)
- Ветеринария и жизнь / Зообизнес / Россельхознадзор: 36 иностранных производителей ветпрепаратов прошли GMP-инспекции. URL: https://vetandlife.ru/sobytiya/rosselhoznadzor-36-inostrannyh-proizvoditelej-vetpreparatov-proshli-gmp-inspekcii/ (дата обращения 04.07.2023)
- ЗооМедВет / Статьи / Зарубежные ветеринарные препараты: перспективы в условиях ограничений. URL: https://zoomedvet.ru/?p=10013 (дата обращения 04.07.2023)
- Известия / Рубрики / Экономика / Загнать в укол: в Госдуме увидели риски дефицита препаратов для животных. URL: https://iz.ru/1494625/mariia-perevoshchikova-natalia-bashlykova/zagnat-v-ukol-v-gosdume-uvideli-riski-defitcita-preparatov-dlia-zhivotnykh (дата обращения 07.2023)
- Евразийский экономический союз / Решение Совета Евразийской экономической комиссии от 03.11.2016 г. № 77 «Об утверждении Правил надлежащей производственной практики Евразийского экономического союза». URL: https://docs.eaeunion.org/docs/ru-ru/01411921/cncd_21112016_77 (дата обращения 04.07.2023)
- ФГБУ «ВГНКИ» / Структура / Отдел инспекции производства на соответствие требованиям надлежащей производственной практики / График проведения инспектирования иностранных производителей на соответствие требованиям надлежащей производственной практики. URL: https://www.vgnki.ru/assets/files/2023/grafik-na-sajt-29062023.pdf (дата обращения 04.07.2023)
- Национальная ветеринарная ассоциация / Российский рынок ветеринарной фармацевтики обещает бурный рост. URL: https://rosvet.org/novosti/rossijskij-rynok-veterinarnoj-farmaczevtiki-obeshhaet-burnyj-rost/ (дата обращения 04.07.2023)
- Ветеринария и жизнь / Зообизнес / В Минсельхоз не поступала информация об уходе с рынка иностранных производителей ветпрепаратов. URL: https://vetandlife.ru/sobytiya/v-minselhoz-ne-postupala-informaciya-ob-uhode-s-rynka-inostrannyh-proizvoditelej-vetpreparatov/ (дата обращения 04.07.2023)
- Ветбиохим / Публикации / Сертификат GMP. URL: https://vetbio.ru/public/news-vbh/320/ (дата обращения 04.07.2023)
- ФГБУ «ВГНКИ» / Пресс-центр / Новости / Заместитель директора ФГБУ «ВГНКИ» Василина Грицюк выступила с докладом на Веткогрессе MVC 2023. URL: https://www.vgnki.ru/zamestitel-direktora-fgbu-vgnki-vasilina-gricyuk-vystupila-s-dokladom-na-vetkongresse-mvc-2023.html (дата обращения 04.07.2023)
- Аналитика Экспо / Все мероприятия SMARTLABFORUM 13 апреля. URL: https://analitikaexpo.com/ru/agenda/sessions/SmartLab3/ALL/ (дата обращения 04.07.2023)
- ФармПром.РФ / Материалы партнеров / Как производить качественную фармпродукцию в условиях турбулентности. URL: https://pharmprom.ru/kak-proizvodit-kachestvennuyu-farmprodukciyu-v-usloviyax-turbulentnosti/ (дата обращения 04.07.2023)
- Минсельхоз России / Департамент ветеринарии / Отраслевая информация / Информация о государственной услуге по аттестации лица, уполномоченного держателем или владельцем регистрационного удостоверения лекарственного препарата для ветеринарного применения. URL: https://mcx.gov.ru/ministry/departments/departament-veterinarii/industry-information/ (дата обращения 07.2023)
- Официальный интернет-портал правовой информации / Приказ Министерства сельского хозяйства Российской Федерации от 10.11.2022 № 795 «Об утверждении Порядка аттестации лица, уполномоченного держателем или владельцем регистрационного удостоверения лекарственного препарата для ветеринарного применения». URL: http://publication.pravo.gov.ru/Document/View/0001202211290041 (дата обращения 04.07.2023)
- ФармПром.РФ / Регуляторы фармрынка / Что нужно знать лицу, уполномоченному держателем или владельцем РУ ветпрепарата. URL: https://pharmprom.ru/chto-nuzhno-znat-licu-upolnomochennomu-derzhatelem-ili-vladelcem-ru-vetpreparata/ (дата обращения 04.07.2023)
- The rules governing medicinal products in the European Union – Volume 4 – EU Guidelines for Good Manufacturing Practice for Medicinal Products for Human and Veterinary Use / Annex 1 – Manufacture of Sterile Medicinal Products. URL: https://health.ec.europa.eu/system/files/2022-08/20220825_gmp-an1_enpdf (дата обращения 04.07.2023)
- Евразийская Академия надлежащих практик / Новости и события / 18 мая на платформе Академии состоится бесплатный вебинар на тему валидации процессов стерилизующей фильтрации. URL: https://gxp-academy.org/about/news_and_events/1234/ (дата обращения 04.07.2023)
- ФГБУ «ВГНКИ» / Отдел инспекции производства на соответствие требованиям надлежащей производственной практики / Полезная информация / Перечень наиболее часто встречающихся несоответствий при инспектировании производителей лекарственных средств для ветеринарного применения. URL: https://www.vgnki.ru/otdel-inspekcii-proizvodstva-na-sootvetstvie-trebovaniyam-nadlezhashhej-proizvodstvennoj-praktiki.html (дата обращения 04.07.2023)
- VIII Всероссийская GMP-конференция. URL: http://gosgmp.ru/ (дата обращения 04.07.2023)
- Евразийская Академия надлежащих практик / Образовательная деятельность Образовательные программы (план) / ПК «Подготовка аудиторов производства лекарственных средств». URL: https://gxp-academy.org/education/courses/podgotovka-auditorov-proizvodstva-lekarstvennykh-sredstv-23-2/ (дата обращения 04.07.2023)
- SCM Pharm / Мероприятия / V Международная конференция Логистика лекарственных средств. URL: https://scmpharm.ru/events/5-offline-conf/#terms (дата обращения 04.07.2023)

