
C 1 сентября этого года вступают в силу From September 1, 2023, amendments to Federal Law No. 61-FZ of 12.04.2010 “On the Circulation of Medicines” introduced by Federal Law No. 317-FZ of 02.07.2021 [1], come into force, and the introduction into civil circulation of a medicinal product for veterinary use imported into the Russian Federation will be carried out if there is a conclusion on the compliance of the manufacturer of medicinal products with the requirements of the Good Manufacturing Practice (GMP) Rules issued by the authorized federal executive body for the manufacturing site of the medicinal product for veterinary use introduced into civil circulation. This review provides information related to the inspection of manufacturers of veterinary medicines for compliance with the GMP Rules requirements which is carried out by specialists of the Federal State Budgetary Institution “The All-Russian State Center for Quality and Standardization of Veterinary Drugs and Feed” (FSBI “VGNKI”) subordinated to Rosselkhoznadzor.
Inspection results
Foreign manufacturers
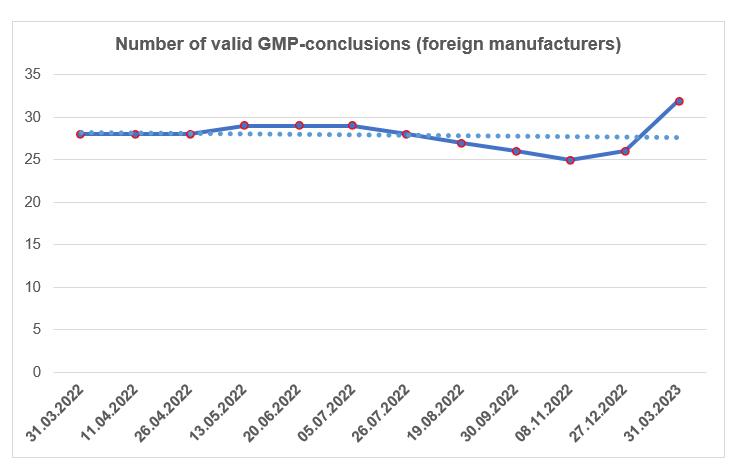
According to the register of conclusions, published on the Rosselkhoznadzor website 31.03.2023 [2], in the first quarter of this year, 7 conclusions on compliance with the GMP Rules requirements were issued. These conclusions were made for sites located in Vietnam, Israel, India, Spain, China, New Zealand and Slovakia.
Up-to-date information is published on the official website of the regulator; the section “Russian and foreign enterprises certified according to the EAEU GMP” [3] posted on the website of the AVPHARM Association has not been updated since the end of last year.
By the end of the first quarter, the number of valid GMP-conclusions has slightly increased.
To date, foreign manufacturers have 32 valid GMP-conclusions. These conclusions were issued both to the sites of the companies that are members of the AVPHARM Association, and to the sites of other foreign manufacturers.
In March of this year, the Russian regulator wrote on its website [4] that some foreign manufacturers of veterinary medicines are withdrawing applications for inspections of their manufacturing sites by Rosselkhoznadzor, which is a standard procedure for world practice, not complicated by any unnecessary requirements.
The AVPHARM Association commented on this message of Rosselkhoznadzor [5]: “The current legal norms do not provide for the possibility of postponing inspections within the framework of the current application; therefore, applicants are periodically forced to withdraw them and submit new ones. As a result of unsuccessful inspections, members of the Association make the necessary changes to the production and control processes and then submit new inspection applications”.
In turn, the FSBI “VGNKI” published the following comment on its website [6]: “Foreign manufacturing companies from unfriendly countries, including those included in AVPHARM, cannot organize a visit by a commission of inspectors (consulates simply do not issue a visa to enter the country where the production site is located; or they cannot logistically organize the visit), and they refuse remote inspections and ask to postpone the inspection, although before that they insisted on using the remote inspection format. After the refusal, manufacturers can again, the very next day, freely apply for inspection”.
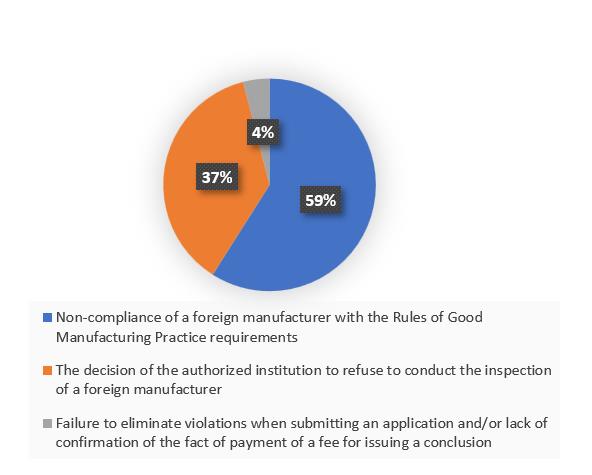
According to the register on the Rosselkhoznadzor website [2], for the entire period of inspection, the basis for 37% of refusals to issue a GMP-conclusion was “the decision of the authorized institution to refuse to conduct the inspection of a foreign manufacturer in case of non-signing of an agreement, at the initiative of a foreign manufacturer or non-payment of the costs associated with the inspection”.
In accordance with the inspection schedule published on the Rosselkhoznadzor website 29.03.2023 [7], 15 inspections of manufacturers of veterinary medicinal products are planned for the second half of 2023, which sites located in Belgium, Hungary, Germany, Greece, Spain, Italy, China, the Netherlands, New Zealand, France and the Czech Republic.
Russian manufacturers
According to the register on the Rosselkhoznadzor website [2], in the first quarter of the current year, 4 GMP-conclusions were issued. These conclusions were obtained by the following manufacturing sites: REPROVET, MATERIA MEDICA HOLDING, Kursk Biofactory – firm BIOK and Plant Medsintez.
To date, domestic manufacturers have 29 valid conclusions. These conclusions were issued both to the sites of manufacturers of veterinary medicines and to the several sites of manufacturers of medicinal products for human use.
Russian manufacturers of medicinal products for veterinary use are increasing their presence in the domestic market. A number of enterprises are engaged in the modernization of existing production and the construction of new production workshops (for example, these are the enterprises of GC VIC, NITA-FARM). At the AVZ enterprise the production on a contract basis of three French medicinal products for veterinary use continues.
In the near future, it is planned to solve the problem of the shortage of Russian vaccines: the largest domestic manufacturers (for example, the Vetbiokhim enterprise) are actively increasing their capacities [8].

Past events and activities
In February, on the federal portal of draft regulatory legal acts (https://regulation.gov.ru/) a draft Order of Rosselkhoznadzor “On approval of the Administrative Regulations of the Federal Service for Veterinary and Phytosanitary Surveillance for the provision of the public service “Issuance of a conclusion on the compliance of a manufacturer of medicinal products for veterinary use with the GMP rules requirements” was posted for public discussion [9].
In March, on the same portal a draft Order of Rosselkhoznadzor “On amendments to the Methodology for calculating the maximum amount of payment for the provision of services for the inspection of manufacturers of medicinal products for veterinary use whose production is carried out outside the Russian Federation for compliance with the GMP rules requirements, for the purpose of issuing conclusions on the compliance of a manufacturer of medicinal products for veterinary use with the GMP rules requirements, approved by the Order of Rosselkhoznadzor dated 07.10.2020 No. 1078″ was posted for public discussion [10].
According to Executive Director of the AVPHARM Association Semen Zhavoronkov, who he shared at the end of last year on the “ZooMedVet” portal [11], due to the peculiarities of the interpretation of certain GMP provisions by the Russian veterinary pharmaceutical inspectorate, on average, one successful inspection accounts for 3-4, ending unsatisfactorily.
Representatives of the FSBI “VGNKI” inspectorate have repeatedly emphasized that foreign manufacturers need to study the EAEU GMP Rules, approved by the Resolution of the EEC Council dated 03.11.2016 No. 77 [12], and conduct audits at the sites for compliance with the requirements of these Rules.
Participation in seminars, webinars and advanced training programs held in Russia can help manufacturers understand the peculiarities of the interpretation by inspectors of certain provisions of the EAEU GMP Rules.
Already in the first quarter of this year, several events took place that could be useful for manufacturers to prepare for the inspection.
In the section “Additional professional education” on the FSBI “VGNKI” website there is a Plan of training events for manufacturers of medicines for veterinary use and feed additives for 2023 [13].
For example, in February, on the basis of the FSBI “VGNKI”, an online seminar was held for internal auditors and employees responsible for organizing and conducting self-inspection at enterprises producing medicinal products for veterinary use. Program content:
- General principles and role of self-inspection
- Internal audit programs and plans
- The procedure for preparing for self-inspection
- Sources and methods of obtaining information
- Classification of nonconformities
- Corrective and preventive actions.
An effectively established self-inspection system becomes an effective tool for ensuring the functioning and improvement of the pharmaceutical quality system, as well as solving many problems regarding the quality of products [14].
In March, on the platform of the Eurasian Academy of Good Practices, the next advanced training course “Training auditors of the production of medicinal products” was held (correspondence form of education using distance learning technologies). Course content:
- GMP requirements for the manufacturing process of medicinal products
- Quality Control. GMP requirements for quality control of medicinal products
- Activities outsourced to another organization (Outsourced Activities)
- Complaints and Product Recall
- Self-inspection. Conducting audits and self-inspections in pharmaceutical production
- Quality Risk Management (GMP Part III, document ICH Q9). Corrective and preventive action system. The system of work with changes, deviations, non-compliances within the framework of GMP.
This course is designed for the following audience: inspectors/auditors (future and/or current), employees of medicinal products manufacturers, employees of industry departments and organizations. As Tatyana Nikolko, Head of Educational Projects, DTDPI FSI “SID & GP” of the Ministry of Industry and Trade of the Russian Federation noted, there are certain requirements for pharmaceutical inspectors/auditors and internal experts of drug manufacturers both in the regulatory legal acts and internal documents of organizations. Without meeting these requirements, it is impossible to become an inspector, auditor, internal expert.
The advanced training course “Training auditors of the production of medicinal products” is also scheduled for September this year [15].
In the first quarter of this year, many webinars were dedicated to the new Annex 1 to EU GMP [16], which will be implemented in the European Union in August this year.
As Leading Qualification Specialist of PQE EAEU Alexander Belinsky noted in the article “New GMP EU Annex 1: from reasoning to specifics”, almost any aspect of sterile production in the updated GMP EU Annex 1 is considered in much more detail than in its previous edition. It is not always aimed at tightening the requirements, rather, on the contrary, quite ample opportunities are often provided to justify certain decisions by reflecting them in the сcntamination сcntrol strategy (CCS). At the same time, key parameters have been identified, the list of which and the frequency of control are now unambiguously fixed and are not subject to discussion regarding the need to fulfill [17].
In the first quarter, the “PHARMSTRATEGY” company held several open expert and consulting webinars, the purpose of which was to understand the updates in the EU regulatory requirements in order to better understand the prospective changes in the EAEU requirements for organizing the manufacture of sterile medicinal products. Expert presentations were devoted to the following topics:
- Modern approaches to confirming the antimicrobial efficacy of disinfectants
- New approaches to the organization of production lines for sterile medicinal products
- A risk-based approach to aseptic process validation
- An approach to developing CCS based on the new PDA guidelines.
The company’s calendar and thematic plan for the first half of 2023 [18] includes other webinars on new requirements for the manufacture of sterile medicinal products in the light of the revision of Annex 1 to EU GMP.
It was possible to take part in some international events remotely.
The French company Elis held several free webinars on Annex 1.
For example, the free webinar “How to comply with Annex 1 in the Cleaning & Disinfection of your Cleanrooms”, organized in January in cooperation with Vileda company, was quite useful. The webinar featured excerpts from the new version of the Annex; how to properly clean and disinfect a cleanroom; demonstrated a sustainable solution that can help the company make savings.
In February, the company held a webinar “Aseptic gowning and behaviour for cleanroom grades A and B”. The new version of Annex 1 focuses intently on personnel control, which includes training in aseptic gowning and behavior for grades A and B, as well as periodic reassessment at least annually. Correct aseptic gowning can greatly reduce the risk of cross-contamination while entering your cleanroom. The last webinar covered all steps of correct aseptic gowning as described in Annex 1 and IEST standards; gowning videos were shown, and each step was analyzed.
In March, another company event took place – “Annex 1 Update & Review webinar”.
In March, PDA Europe organized the free webinar “2023 PDA Develop impactful Training in Times of the New EU GMP Annex 1”. The revised Annex 1 requires significant adjustments for manufacturers of sterile products, including training cleanroom personnel. At the same time, advanced manufacturing procedures require highly qualified personnel and therefore advanced training. This webinar provided a practical guide on creating an impactful and Annex 1 compliant training curriculum as a crucial part of a CCS. The webinar focused on using VR Simulators as immersive technologies, which are employed in a risk-based approach to enhance the effectiveness and impact of training. The topics covered included:
- How to create risk-based learning objectives
- How to create standardized VR Simulator lessons and link them to site-specific processes
- How to create meaningful employee assessments
- How to objectively evaluate behavior using VR in line with APS.
Upcoming events and activities
In April, the XXXI Moscow International Veterinary Congress MVC 2023 will be held. On April 12, the section “Business and government” is scheduled within the framework of the congress, where representatives of the Department of Veterinary Medicine of the Ministry of Agriculture of Russia (“Medication support for veterinary activities in modern conditions”) and FGBU “VGNKI” (“Introduction into civil circulation of medicinal products for veterinary use”) are expected to speak [19].
Before the introduction into civil circulation of each batch of a medicinal product for veterinary use imported into the Russian Federation, the importing organization will have to submit to Rosselkhoznadzor a confirmation of the compliance of the medicinal product with the requirements established during its state registration, from the person authorized by the holder or owner of the marketing authorization of a medicinal product for veterinary use.
You can read more about “person authorized by the marketing authorization holder or owner” here (RU).
On April 20, Moscow will host the XII International Pharmaceutical Forum PharmPRO 2023. This is a platform for live communication and exchange of experience between drug manufacturers, government officials and industry experts. The forum will include a session “How to ensure stable production of high-quality pharmaceutical products in the face of constant changes?“, where, in particular, the issues of the work of pharmaceutical companies with their suppliers will be considered [20].
Printed packaging materials are considered critical to product compliance and should be given special attention as required by the GMP Rules. They must be purchased from approved, audited suppliers.
You can read about the audit of suppliers of printed secondary packaging materials here (RU).
Manufacturers are encouraged to take an active part in GM(D)P-related events and activities and prepare more thoroughly for GMP inspections.
The material presented was prepared using data relevant to 02.04.2023 In case of new or additional data the article can be updated.
The Federal State Budgetary Institution “The All-Russian State Center for Quality and Standardization of Veterinary Drugs and Feed”, FSBI “VGNKI” is an institution authorized to conduct inspections of manufacturers of medicinal products for veterinary use manufactured outside the Russian Federation for compliance with the GMP rules requirements
The Association of Veterinary Pharmaceutical Manufacturers AVFARM is an association representing the interests of leading international pharmaceutical companies in the Russian Federation – manufacturers of medicinal products for veterinary use (MSD Animal Health, Elanco, Zoetis and Boehringer Ingelheim)
The Parenteral Drug Association (PDA) is an international non-profit industry trade group for pharmaceutical and biopharmaceutical manufacturers
The Institute of Environmental Sciences and Technology (IEST) is the leading technical, nonprofit membership association that connects professionals who deal with controlled environments
References:
- Электронный фонд правовых и нормативно-технических документов / Федеральный закон от 02.07.2021 г. № 317-ФЗ «О внесении изменений в Федеральный закон «Об обращении лекарственных средств». URL: https://docs.cntd.ru/document/607142404 (дата обращения 02.04.2023)
- Россельхознадзор / Деятельность / Госуслуги / Выдача заключения о соответствии производителя (иностранного производителя) лекарственных средств для ветеринарного применения требованиям правил надлежащей производственной практики / Дополнительная информация для заявителя / Государственный реестр заключений о соответствии производителя (иностранного производителя) лекарственных средств для ветеринарного применения требованиям правил надлежащей производственной практики. URL: https://fsvps.gov.ru/ru/gosuslugi/zaklyuchenie-o-sootvetstvii (дата обращения 02.04.2023)
- АВФАРМ / Аналитика / Предприятия с GMP. URL: https://analytics.avpharm.ru/gmp (дата обращения 02.04.2023)
- Россельхознадзор / Новости / Главные новости / Иностранные производители ветеринарных препаратов для животных отказываются от проведения инспектирования своих заводов. URL: https://fsvps.gov.ru/ru/fsvps/news/216879.html (дата обращения 02.04.2023)
- ФармПром.РФ / Новости фармацевтической отрасли / Зарубежные производители ветпрепаратов, входящие в АВФАРМ не отказываются от проверок. URL: https://pharmprom.ru/zarubezhnye-proizvoditeli-vetpreparatov-vxodyashhie-v-avfarm-ne-otkazyvayutsya-ot-proverok/ (дата обращения 02.04.2023)
- ФГБУ «ВГНКИ» / Пресс-центр / Новости / ФГБУ «ВГНКИ» прояснило ситуацию о проверках зарубежных производителей ветпрепаратов. URL: https://www.vgnki.ru/fgbu-vgnki-proyasnilo-situaciyu-o-proverkah-zarubezhnyh-proizvoditelej-vetpreparatov.html (дата обращения 02.04.2023)
- Россельхознадзор / Деятельность / Госуслуги / Выдача заключения о соответствии производителя (иностранного производителя) лекарственных средств для ветеринарного применения требованиям правил надлежащей производственной практики / Дополнительная информация для заявителя / График инспектирования производителей. URL: https://fsvps.gov.ru/ru/gosuslugi/zaklyuchenie-o-sootvetstvii (дата обращения 02.04.2023)
- Союз предприятий зообизнеса / Новости / В «Деловой России» обсудили дефицит ветпрепаратов и специализированных кормов. URL: http://www.spzoo.ru/cntnt/default/n7853.html (дата обращения 02.04.2023)
- Федеральный портал проектов нормативных правовых актов / Проект Приказа Россельхознадзора «Об утверждении Административного регламента Федеральной службы по ветеринарному и фитосанитарному надзору по предоставлению государственной услуги «Выдача заключения о соответствии производителя лекарственных средств для ветеринарного применения требованиям правил надлежащей производственной практики». URL: https://regulation.gov.ru/p/135840 (дата обращения 02.04.2023)
- Федеральный портал проектов нормативных правовых актов / Проект Приказа Россельхознадзора «О внесении изменений в Методику расчета предельного размера платы за оказание услуги по инспектированию производителей лекарственных средств для ветеринарного применения, производство которых осуществляется за пределами Российской Федерации, на соответствие требованиям правил надлежащей производственной практики в целях выдачи заключений о соответствии производителя лекарственных средств для ветеринарного применения требованиям правил надлежащей производственной практики, утвержденную Приказом Россельхознадзора от 07.10.2020 г. № 1078». URL: https://regulation.gov.ru/p/136869 (дата обращения 02.04.2023)
- ЗооМедВет / Статьи / Закон есть закон / Регулирование обращения ветеринарных лекарственных препаратов: текущий статус.: https://zoomedvet.ru/?p=8686 (дата обращения 02.04.2023)
- Евразийский экономический союз / Решение Совета Евразийской экономической комиссии от 03.11.2016 г. № 77 «Об утверждении Правил надлежащей производственной практики Евразийского экономического союза». URL: https://docs.eaeunion.org/docs/ru-ru/01411921/cncd_21112016_77 (дата обращения 02.04.2023)
- ФГБУ «ВГНКИ» / Образование / Дополнительное профессиональное образование / Планы мероприятий/ План обучающих мероприятий для производителей лекарственных препаратов для ветеринарного применения и кормовых добавок ФГБУ “ВГНКИ” на 2023 год. URL: https://www.vgnki.ru/assets/files/plan-2023-proizvoditeli-ls(1).pdf (дата обращения 02.04.2023)
- ФГБУ «ВГНКИ» / Пресс-центр / Новости / В ФГБУ «ВГНКИ» пройдет семинар по организации самоинспекции на фармацевтическом предприятии. URL: https://www.vgnki.ru/v-fgbu-vgnki-projdet-seminar-po-organizacii-samoinspekcii-na-farmacevticheskom-predpriyatii.html (дата обращения 02.04.2023)
- Евразийская Академия надлежащих практик / Образовательная деятельность Образовательные программы (план) / ПК «Подготовка аудиторов производства лекарственных средств». URL: https://gxp-academy.org/education/courses/podgotovka-auditorov-proizvodstva-lekarstvennykh-sredstv-23-2/ (дата обращения 02.04.2023)
- The rules governing medicinal products in the European Union – Volume 4 – EU Guidelines for Good Manufacturing Practice for Medicinal Products for Human and Veterinary Use / Annex 1 – Manufacture of Sterile Medicinal Products. URL: https://health.ec.europa.eu/system/files/2022-08/20220825_gmp-an1_enpdf (дата обращения 02.04.2023)
- Национальный фармацевтический журнал / Эксперт / Новое приложение 1 GMP EU: от рассуждений к конкретике. URL: https://npjnews.com/eksperts/novoe-prilozhenie-1-gmp-eu-ot-rassuzhdenij-k-konkretike/ (дата обращения 04.2023)
- Фармстратегия / Отчеты / Календарно-тематический план на первое полугодие 2023. URL: https://goodpractices.ru/upload/iblock/817/pohkvsiso8vjvfjjw7gg0ix63acdngbv/2022_12_26_KTP_2023_mail.pdf (дата обращения 02.04.2023)
- XXXI Московский международный ветеринарный конгресс / Мероприятия / Программа секций / XXXI Ветконгресс / Первый день / Бизнес и власть Бизнес и власть. URL: https://vetcongress.ru/page/593 (дата обращения 02.04.2023)
- PharmPRO / XII фармацевтический форум PharmPRO. URL: https://events.pharmpro.pro/forum-2023 (дата обращения 02.04.2023)

