
The review for the third quarter of 2024 provides information on the inspection of manufacturers of veterinary medicines for compliance with the requirements of the Good Manufacturing Practice (GMP). These inspections are carried out by specialists of the Inspection Body of the Federal State Budgetary Institution “The All-Russian State Center for Quality and Standardization of Veterinary Drugs and Feed” (FSBI “VGNKI”).
Foreign manufacturers
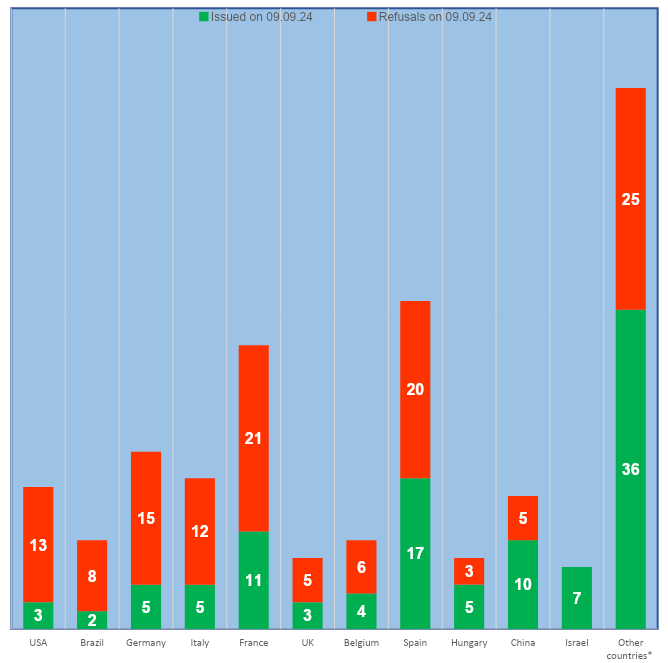
According to the register of conclusions, published on the Rosselkhoznadzor website 09.09.2024 [1], in the third quarter of this year, 4 conclusions on compliance with the GMP Rules requirements were issued. These conclusions were made for sites located in Bulgaria, Germany and China.
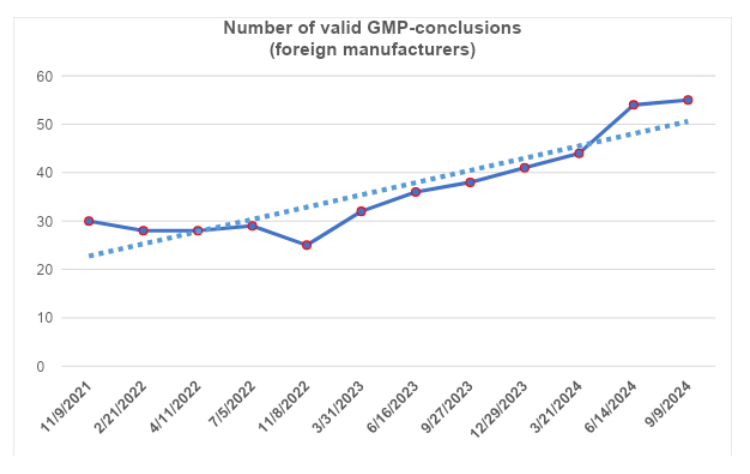
According to the register [1], by the end of the third quarter, the number of valid GMP-conclusions remained at the same level (the validity period of four conclusions expired). To date, foreign manufacturers have 54 valid GMP-conclusions.
The executive director of the AVPHARM association, Semen Zhavoronkov, commented on the development of the situation [2]: “Now more certificates [GMP-conclusions] have been issued, there is some progress in terms of the number and share of inspections that end positively, but in order to translate this into the availability of medicines, the dynamics must be maintained.”
The largest number of conclusions (more than 60%) are from production sites located in China, Spain, France, Israel, Italy, Germany and the USA. At the same time, the share of valid GMP-conclusions issued to sites from countries friendly to Russia has increased and amounts to 37%. These conclusions were made for sites located in Argentina, Belarus, Brazil, Vietnam, Israel, India, China and Turkey. The number of inspections carried out at manufacturing sites in China continues to increase. In the third quarter, GMP-conclusions were issued to two Chinese sites, including the manufacturer of antigens of the avian influenza virus, Newcastle disease of birds, circovirus and pneumonia of pigs [3]. In July of this year, on the sidelines of the SCO summit in Astana, a meeting was held between Russian President Vladimir Putin and Chinese President Xi Jinping. During the talks, the Russian leader noted [4]: “Trade is growing. We noted this during my visit to the People’s Republic of China. And today we can confirm this once again. We have noted positive dynamics in the first half of this year.”
In accordance with the inspection schedule published on the VGNKI website 17.09.2024 [5], 6 inspections of manufacturers of veterinary medicinal products are planned for the fourth quarter of 2024, which sites located in Austria, the UK, China, France and Croatia.
Russian manufacturers
According to the register on the Rosselkhoznadzor website [1], 3 GMP-conclusions were issued in the third quarter of this year. These conclusions were received by the manufacturing sites of FARMBIOMEDSERVIS, BIOTSENTR and the FGBI ARRIAH.
The production volumes of vaccines developed by the FGBI ARRIAH are growing annually. Compared to last year, production output has increased more than threefold and currently exceeds 8 million doses. Recently, the FGBI ARRIAH has agreed on scientific and production cooperation with the Korean pharmaceutical company Green Cross. This concerns the organization of vaccine production using Korean technologies at the Russian institute [6].
To date, domestic manufacturers have 28 valid conclusions.
Past events and activities
In the third quarter of this year, several events and activities took place that might be of interest to manufacturers preparing for inspection.
In August, the IX All-Russian GMP Conference was held in Ufa. The conference was organized by the Ministry of Industry and Trade of Russia together with the FSI “SID & GP”. The great annual interest in the GMP conference from foreign partners allows for an international dialogue on pressing issues of intercountry cooperation in the field of medicines production. This year alone, representatives from 23 countries, as well as the WHO, took part in the event in person and online [7].
In September, the State Duma rejected draft Law No. 555529-8 “On suspension of Part 3 of Article 52-2 of the Federal Law “On the Circulation of Medicines” [8] (draft law on suspension of the obligation to obtain a GMP-conclusion for veterinary medicinal products imported from abroad).
In the same month, the Department of Sanitary, Phytosanitary and Veterinary Measures of the Eurasian Economic Commission (EEC) held an open event at the Chamber of Commerce and Industry of the Russian Federation (RF CCI) to answer questions from businesses in connection with the deepening integration of the common Eurasian market for veterinary medicines [9]. The technical organizers of the event were the Union of Animal Industry Enterprises (SPZ) and the AVPHARM association with the support of the RF CCI Committee for the development of the agro-industrial complex. The moderator of the discussion panel was Evgeniya Alekseeva, advisor to the veterinary measures department of the Department of sanitary, phytosanitary and veterinary Measures of the EEC. Among other things, she said that the first application for a joint pharmaceutical inspection of the manufacturer had recently been submitted to the authorized body of the Republic of Belarus. This inspection for compliance with the requirements of the EAEU GMP Rules, approved by the Resolution of the EEC Council dated 03.11.2016 No. 77 [10], was planned to be carried out in accordance with the Rules for conducting pharmaceutical inspections, which are Appendix No. 26 to the “Rules for the regulation of the circulation of veterinary medicinal products in the customs territory of the EAEU”, approved by the Resolution of the EEC Council dated 21.01.2022 No. 1 [11].
One of the positive changes in the field of GMP-inspections was specifically noted at the event – now, where nonconformities have been identified during inspection, a response with an attached plan of corrective and preventive actions (CAPA-plan), and a report on its implementation with materials confirming the fact of their implementation must be sent to the authorized body that organized the inspection no later than 80 calendar days from the date of receipt of the inspection report (in accordance with the Resolution of the EEC Council dated 22.04.2024 No. 36 “On Amendments to the Rules for the regulation of the circulation of veterinary medicinal products in the customs territory of the EAEU” [12]).
Upcoming events and activities
On October 18, the VI International Conference “Medicinal products logistics” [13] will be held in Moscow. The conference will cover more than 30 topics and will announce the results of the third all-Russian survey of pharmaceutical logistics and quality SCM Pharm Survey 2024, and participants will receive the GDP Review 5 practical collected articles.
Among other things, the conference program includes an event entitled “Main trends in the transportation and storage of veterinary products. Labeling and other challenges in 2024”, where the following topics are planned to be discussed:
- Current state of the veterinary medicines market and forecasts for further development
- Modern approach to transportation and storage of veterinary products. Pros and cons of outsourcing these processes
- Import, customs clearance and labeling of veterinary medicines. What difficulties arise? Answers to the main questions of manufacturers.
The event is planned to be attended by Yulia Kalinina, head of the Department for the organization of state supervision in the sphere of circulation of medicines for veterinary use of the State veterinary supervision department of Rosselkhoznadzor (remotely via video link); Vilnur Shagiakhmetov, project manager of the Pharma product group of CRPT; Harold Vlasov, managing director of the pharmaceutical 3PL operator NC Logistic.
It should be recalled that since September of this year, the EAEU GDP Rules, approved by the Resolution of the EEC Council dated 03.11.2016 No. 80 [14], also apply to medicinal products for veterinary use (in accordance with the RF Government Decree No. 756 of 01.06.2024 “On Amendments to RF Government Decree of March 31, 2022 No. 547” [15]). You can read about changes in the Good Distribution Practice (GDP) Rules for veterinary medicines here.
Also in September, the Rules for labeling medicinal products for veterinary use with identification means, approved by the RF Government Decree No. 675 of 27.05.2024 “On Approval of the Rules for labeling medicinal products for veterinary use with identification means and the specifics of implementing the state information system for monitoring the circulation of goods subject to mandatory labeling with identification means, with respect to medicinal products for veterinary use” came into force [16]. An important point mentioned in paragraph 3 of the Decree is the exception for vaccines that have a storage and transportation temperature of minus 60 degrees Celsius or lower. You can read about the specifics of veterinary medicines stored in Dewar vessels here.
The annual Eurasian Validation Conference will be held in Moscow on October 22-24 [17]. This is an event with educational potential aimed at discussing current theoretical and practical issues in the field of risk analysis, qualification and validation to confirm Good Practices. Over the course of three days, participants will listen to presentations by experts, take part in interesting discussions, and informal communication with conference participants dedicated to modern approaches to validation, practical methods and technologies in this area.
In November, Moscow will host the leading international exhibition of equipment, raw materials and technologies for pharmaceutical production in Russia and the EAEU countries, Pharmtech & Ingredients 2024.
On November 21, for the first time, the exhibition will host a round table dedicated to the veterinary medicines market as part of the business program – “The market of medicinal products for veterinary use: its features, problems and prospects” [18]. The speakers at the event are planned to be Vladimir Subbotin, deputy director of the Department for sanitary, phytosanitary and veterinary measures of the EEC; Vilnur Shagiakhmetov, project manager of the Pharma product group of CRPT; Semen Zhavoronkov, executive director of the AVPHARM association.
The National Veterinary Association (NVA) became a partner of the event. The Association monitored the intermediate results of the experiment on labeling with identification means of veterinary medicines. To meet the tight deadlines for preparation for mandatory labeling, an analysis was regularly conducted of the number of contracts concluded for the supply of equipment for labeling by manufacturers, the number of manufacturers already equipped with equipment for labeling, and the activity of the Association’s participants in terms of working out issues of labeling veterinary medicines [19].
Manufacturers are encouraged to take an active part in GM(D)P-related events and activities and prepare more thoroughly for GMP inspections.
| The material presented was prepared using data relevant to 27.09.2024. In case of new or additional data the article can be updated. |
The Federal State Budgetary Institution “The All-Russian State Center for Quality and Standardization of Veterinary Drugs and Feed”, FSBI “VGNKI” is an institution authorized to conduct inspections of manufacturers of medicinal products for veterinary use manufactured outside the Russian Federation for compliance with the GMP Rules requirements
The National Veterinary Association, NVA is a non-profit organization that is a collaboration of key biopharmaceutical companies
The Union of Animal Industry Enterprises (SPZ) – a non-profit organization that brings together manufacturers and distributors of veterinary drugs, pet food and feed additives, apparel and accessories, hygiene and beauty care products, wholesalers, retailers, veterinary clinics, specialized media, associations and pet breeders
The Association of Veterinary Pharmaceutical Manufacturers AVPHARM is an association representing the interests of leading international pharmaceutical companies in the Russian Federation – manufacturers of medicinal products for veterinary use (MSD Animal Health, Zoetis and Boehringer Ingelheim)
Reference:
- Россельхознадзор / Деятельность / Госуслуги / Выдача заключения о соответствии производителя (иностранного производителя) лекарственных средств для ветеринарного применения требованиям правил надлежащей производственной практики / Дополнительная информация для заявителя / Государственный реестр заключений о соответствии производителя (иностранного производителя) лекарственных средств для ветеринарного применения требованиям правил надлежащей производственной практики. URL: https://fsvps.gov.ru/ru/gosuslugi/zaklyuchenie-o-sootvetstvii (дата обращения 27.09.2024)
- АВФАРМ / Новости АВФАРМ / Динамика ввода в гражданский оборот отражает дефицит привычных ветпрепаратов в продаже. URL: https://avpharm.ru/news/market-release-dynamics/ (дата обращения 27.09.2024)
- ФГБУ «ВГНКИ» / Пресс-центр / Новости / Подведомственное Россельхознадзору ФГБУ «ВГНКИ» провело 10 проверок иностранных площадок по производству ветеринарных препаратов с 2 мая по 9 июля 2024 года. URL: https://www.vgnki.ru/podvedomstvennoe-rosselhoznadzoru-fgbu-vgnki-provelo-10-proverok-inostrannyh-ploshhadok-po-proizvodstvu-veterinarnyh-preparatov-s-2-maya-po-9-iyulya-2024-goda.html (дата обращения 27.09.2024)
- РИА Новости / Путин отметил положительную динамику во взаимной торговле с Китаем. URL: https://ria.ru/amp/20240703/putin-1957161695.html (дата обращения 27.09.2024)
- ФГБУ «ВГНКИ» / Структура / Отдел инспекции производства на соответствие требованиям надлежащей производственной практики / График проведения инспектирования иностранных производителей на соответствие требованиям надлежащей производственной практики. URL: https://www.vgnki.ru/assets/2024/files/grafik-na-sajt-17092024.pdf (дата обращения 27.09.2024)
- Ветеринария и жизнь / Зообизнес / ВНИИЗЖ будет выпускать вакцины для свиней и птиц с корейской фармкомпанией. URL: https://vetandlife.ru/pet-business/vniizzh-budet-vypuskat-vakciny-dlya-svinej-i-ptic-s-korejskoj-farmkompaniej/ (дата обращения 27.09.2024)
- IX Всероссийская GMP-конференция / Новости / Подведены итоги IX Всероссийской GMP-конференции с международным участием. URL: https://gosgmp.ru/2024/09/02/podvedeny-itogi-ix-vserossijskoj-gmp-konferenczii-s-mezhdunarodnym-uchastiem/ (дата обращения 27.09.2024)
- СОЗД / Объекты законотворчества / Законопроект № 555529-8 «О приостановлении действия части 3 статьи 52-2 Федерального закона «Об обращении лекарственных средств». URL: https://sozd.duma.gov.ru/bill/555529-8 (дата обращения 27.09.2024)
- ТПП РФ / Новости / Практику применения Правил регулирования обращения ветеринарных лекарственных средств в ЕАЭС обсудили в ТПП РФ. URL: https://news.tpprf.ru/ru/news/6036576/ (дата обращения 27.09.2024)
- Евразийский экономический союз / Решение Совета Евразийской экономической комиссии от 03.11.2016 г. № 77 «Об утверждении Правил надлежащей производственной практики Евразийского экономического союза». URL: https://docs.eaeunion.org/docs/ru-ru/01411921/cncd_21112016_77 (дата обращения 27.09.2024)
- КонсультантПлюс / Решение Совета Евразийской экономической комиссии от 21.01.2022 N 1 (ред. от 22.04.2024) “О Правилах регулирования обращения ветеринарных лекарственных средств на таможенной территории Евразийского экономического союза”. URL: https://www.consultant.ru/document/cons_doc_LAW_409228/ (дата обращения 27.09.2024)
- КонсультантПлюс / Решение Совета Евразийской экономической комиссии от 22.04.2024 N 36 “О внесении изменений в Правила регулирования обращения ветеринарных лекарственных средств на таможенной территории Евразийского экономического союза”. URL: https://www.consultant.ru/document/cons_doc_LAW_476980/ (дата обращения 27.09.2024)
- SCM Pharm / Мероприятия / VI Международная конференция Логистика лекарственных средств. URL: https://scmpharm.ru/events/6-offline-conf/ (дата обращения 27.09.2024)
- Евразийский экономический союз / Решение Совета Евразийской экономической комиссии от 03.11.2016 г. № 80 «Об утверждении Правил надлежащей производственной практики Евразийского экономического союза». URL: https://docs.eaeunion.org/docs/ru-ru/01411930/cncd_21112016_80 (дата обращения 27.09.2024)
- Официальный интернет-портал правовой информации / Постановление Правительства Российской Федерации № 756 от 01.06.2024 «О внесении изменений в Постановление Правительства Российской Федерации от 31 марта 2022 года № 547». URL: http://publication.pravo.gov.ru/document/0001202406030030?index=2 (дата обращения 27.09.2024)
- Официальный интернет-портал правовой информации / Постановление Правительства Российской Федерации от 27.05.2024 № 675 «Об утверждении Правил маркировки лекарственных препаратов для ветеринарного применения средствами идентификации и особенностях внедрения государственной информационной системы мониторинга за оборотом товаров, подлежащих обязательной маркировке средствами идентификации, в отношении лекарственных препаратов для ветеринарного применения». URL: http://publication.pravo.gov.ru/document/0001202405310045 (дата обращения 27.09.2024)
- Евразийская конференция по валидации. URL: https://validconf.ru/ (дата обращения 27.09.2024)
- Pharmtech & Ingredients. Деловая программа / Деловая программа 2024. URL: https://pharmtech-expo.ru/ru/agenda/business-program2024/ (дата обращения 27.09.2024)
- НВА / Новости / Производители лекарственных средств для ветеринарного применения с 1 сентября 2024 года переходят на маркировку «Честный знак». URL: https://rosvet.org/novosti/proizvoditeli-lekarstvennyh-sredstv-dlya-veterinarnogo-primeneniya-s-1-sentyabrya-2024-goda-perehodyat-na-markirovku-chestnyj-znak/ (дата обращения 27.09.2024)

