
On September 1 of this year, the amendments to Federal Law No. 61-FZ of 12.04.2010 “On the Circulation of Medicines” introduced by Federal Law No. 317-FZ of 02.07.2021 [1] came into force. In this regard, the introduction into civil circulation of each batch of a medicinal product for veterinary use imported into the Russian Federation should be carried out if only there is a conclusion on the compliance of the manufacturer of medicinal products with the requirements of the Good Manufacturing Practice (GMP) Rules issued by Rosselkhoznadzor for each manufacturing site. This review provides information on the inspection of manufacturers of veterinary medicines for compliance with the GMP Rules requirements which is carried out by specialists of the Federal State Budgetary Institution “The All-Russian State Center for Quality and Standardization of Veterinary Drugs and Feed” (FSBI “VGNKI”).
Inspection results
Foreign manufacturers
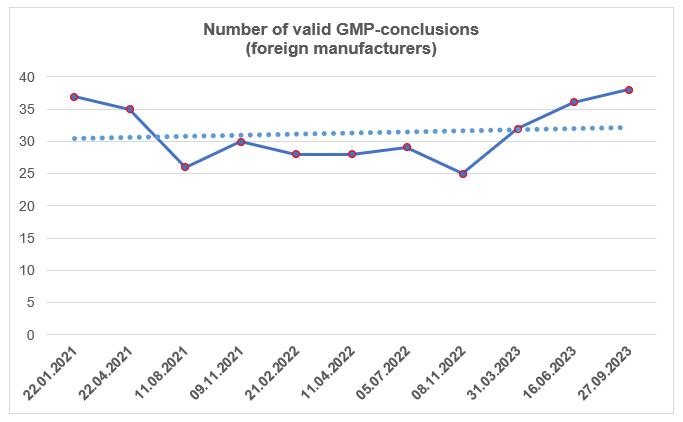
According to the register of conclusions, published on the Rosselkhoznadzor website 27.09.2023 [2], in the third quarter of this year, 3 conclusions on compliance with the GMP Rules requirements were issued. These conclusions were made for sites located in Belarus, Germany and New Zealand.
By the end of the third quarter, the number of valid GMP-conclusions increased slightly again.
To date, foreign manufacturers have 38 valid GMP-conclusions. Four of these conclusions will expire before the end of 2023.
According to updated data, in 2022 the percentage of refusals to issue GMP-conclusions to foreign VMP manufacturers amounted to 58%.
Last year, the FSBI “VGNKI” inspectors conducted 42 inspections of foreign manufacturers. As a result of the inspections, it was found that 24 manufacturing sites do not comply with the GMP Rules requirements, including 13 sites for the production of pharmacological veterinary medicines, 3 sites for the production of vaccines and 8 sites for the production of both types of medicinal products [3].
In accordance with the inspection schedule published on the VGNKI website 19.09.2023 [4], 20 inspections of manufacturers of veterinary medicinal products are planned for the fourth quarter of 2023, which sites located in Brazil, Hungary, Germany, Israel, Spain, China, the Netherlands, Portugal, Romania, the USA and France.
Russian manufacturers
According to the register on the Rosselkhoznadzor website [2], in the third quarter of the current year, a GMP conclusion was issued to the NPF VIC manufacturing site.
The VIC Group of Companies is not only the largest veterinary manufacturer in the CIS, but is also one of the 25 largest veterinary pharmaceutical companies in the world. Recently, the Executive director of the VIC Group of Companies, Sergei Kasparyants, told the NTV television crew about whether there is any need to fear a reduction in the range of medicines for animals in the near future: “I can say with confidence that 99% of veterinary pharmaceuticals will be replaced by domestic medicines that are similar in effectiveness and safety in 1-2 years” [5].
To date, domestic manufacturers have 29 valid conclusions. One of these conclusions will expire before the end of 2023.
Past events and activities
In the third quarter of this year, several events took place that could be useful for manufacturers to prepare for the inspection.
In July, the FSBI “VGNKI” hosted an advanced training course for the training of person authorized by the holder or owner of the marketing authorization for a medicinal product for veterinary use [6]. During the training, a wide range of issues were considered:
- regulatory framework and procedure for introduction immunobiological and pharmaceutical veterinary medicines into civil circulation;
- the powers of the federal executive authorities in the circulation of medicinal products for veterinary use;
- requirements of the registration dossier;
- rules for issuing test reports on the conformity of a batch of immunobiological medicinal product;
- basic requirements for the destruction of confiscated falsified, substandard and counterfeit medicinal products;
- sampling methods for veterinary medicines;
- personnel management;
- production and quality control rules;
- complaints and product recall;
- premises, utilities and equipment;
- general principles of self-inspection;
- corrective and preventive actions.
As a result of passing the attestation, participants could receive certificates of professional development of the established sample.
In accordance with the Order of the Ministry of Agriculture of Russia dated 10.11.2022 No. 795 [7], which entered into force, the test tasks will include fifty questions concerning the requirements of the GMP Rules, as well as the requirements for the registration dossier for a medicinal product for veterinary use.
In the same month, the FSBI “VGNKI” hosted a seminar on “Implementation of Good Distribution Practice (GDP) for medicinal products for veterinary use (GDP)” [8].
GDP Rules for medicinal products for veterinary use came into force on September 1 this year and will be valid until March 12, 2024 [9].
In July, the Center for Strategic Research (CSR) hosted a round table on the topic “Effects of regulating the introduction of veterinary medicinal products into circulation”. During the event, the results of an analysis conducted by the CSR on the socio-economic effects of the procedure for introducing veterinary medicinal products into civil circulation in the Russian Federation were presented.
The compilers of the analysis noted that among the most common violations identified as a result of inspections are, first of all, inconsistencies associated with the absence or incompleteness of the necessary supporting documentation or with the absence of separate control and verification procedures at the enterprise.
The largest share of refusals in 2017-2023 occurred as a result of the non-compliance of VMP manufacturer with the GMP Rules requirements, while more than 30% – as a result of the refusal of the inspection or non-payment of the inspection on time.
Among the key difficulties, representatives of VMP manufacturers also noted the implementation of the corrective and preventive action plan (CAPA-plan) within the prescribed period of 60 working days. This is due both to the fact that individual inconsistencies associated with the repair or re-equipment of production require more time to eliminate, and the fact that with significant changes in production processes, it is necessary to coordinate and take into account these changes with the regulatory authorities of all countries whose markets the manufacturer supplies its products [10].
In August, information was published on the website of the AVPHARM association that Elanco Rus LLC was leaving AVPHARM [11]. Commenting on the decision, Elanco added that “the internal focus of the company and its Russian representative office Elanco Rus LLC will be aimed at improving manufacturing sites and bringing them into compliance with the requirements of the Russian regulator“.
In September, the 2nd China-Russia Forum on Biopharmaceutical Technologies and Business Cooperation was held in Moscow. The event was organized by Chengdu Yingde Biological Pharmaceutical Equipment Co., Ltd. and SinoPharmtech. The main goal of the forum is to develop a mutually beneficial partnership between Russia and China in the fields of biopharmaceuticals and technology transfer. Leaders of the Chinese biopharmaceutical market presented their cases, including Pulike Biological Engineering, which is mainly engaged in research and development, production and marketing of veterinary biological products [12].

Let us recall that last year in China new GMP standard requirements were introduced at enterprises operating in the production of medicinal products for veterinary use. All enterprises that did not pass the inspection before June 1, 2022 were required to suspend the production of veterinary medicines until confirmation of the relevant requirements was received [13].
According to the results of Russian inspections conducted in 2018-2022, manufacturing sites of VMP manufacturers located in China received less than 30% of refusals to issue GMP-conclusions, which is a fairly good indicator.

In September, the VIII All-Russian GMP Conference with international participation, organized by the Ministry of Industry and Trade of Russia together with the FSI “SID & GP”, was held in Ekaterinburg. The motto of this year’s GMP conference is “GMP: good quality in modern conditions”. Director of the FSI “SID & GP” Vladislav Shestakov noted that this slogan reflects the agenda that is relevant for participants in the pharmaceutical industry: “Proper quality in the production of medicinal products is the key to the safety of the citizens of our country. And compliance with regulatory, production requirements and standards in modern conditions gives us the opportunity to move towards import substitution, as well as the active development of exports to new and promising regions for us. In the context of a multipolar world that has been taking shape over recent years, we remain open to intercountry and international cooperation”. [14].
Upcoming events and activities
On October 3, the “PHARMSTRATEGY” company will hold a master class on the topic “Topical issues of inspection of the manufacture of sterile medicinal products. The inspector’s view” [15]. Key topics and concepts:
- inspection of the manufacture of sterile medicinal products;
- Annex 1 to EAEU GMP [16];
- quality risk assessment;
- identified nonconformities.
Until October 10, on the platform of the Eurasian Academy of Good Practices, the next advanced training course “Training auditors of the production of medicinal products” is being held (correspondence form of education using distance learning technologies). Upon completion of training, participants are issued a certificate of professional development of the established sample [17].
On October 20, Moscow will host the V International Conference “Medicinal products logistics” [18]. At the conference, the results of the voting of 339 experts of the jury of the SCM Pharm Award 2023 will be announced, participants will receive the GDP Review 4 practical collected articles.
In particular, the conference will host an interactive discussion “Digital transformation of validation and qualification processes – as part of the Pharma 4.0™ concept”. The moderators of the discussion will be Oxana Pryanichnikova – General Director of PQE CIS, Deputy Director of ISPE EAEU and Alexander Belinsky – Technical Director of PQE CIS, member of the ISPE EAEU Working Group.
The annual Eurasian Validation Conference will be held in Moscow on October 24-26. This is an event with educational potential aimed at discussing current theoretical and practical issues in the field of risk analysis, qualification and validation to confirm Good Practices [19].
New challenges within the EAEU
In less than six months, the new “Rules for the regulation of the circulation of veterinary medicinal products in the customs territory of the EAEU”, approved by the Resolution of the EEC Council dated 21.01.2022 No. 1 [20], will come into force. Appendix 26 to them – Rules for conducting pharmaceutical inspections – establish a common procedure for conducting pharmaceutical inspections of the manufacture of veterinary medicinal products for compliance with the EAEU GMP Rules requirements. According to the current version of the document:
- Where nonconformities have been identified during inspection, a response with an attached plan of corrective and preventive actions (CAPA-plan), and a report on its implementation with materials confirming the fact of their implementation must be sent to the authorized body that organized the inspection no later than 30 calendar days from the date of receipt of the inspection report
- GMP-certificate is issued by the authorized body provided that the inspected entity in the field of circulation of veterinary medicinal products eliminates all critical and major nonconformities, as well as other nonconformities, if, when considered together, they represent major nonconformities
- The procedure for conducting a pharmaceutical inspection using means of remote interaction (remote inspection) is not yet described in the Rules.
It should be noted that in August of this year employees of the FSBI “VGNKI” conducted a refresher course for specialists of the National Reference Center for Veterinary Medicine of the Committee for Veterinary Control and Supervision of the Ministry of Agriculture of the Republic of Kazakhstan. The course program was devoted to the organization of production and quality control of medicinal products for veterinary use [21].
Manufacturers are encouraged to take an active part in GM(D)P-related events and activities and prepare more thoroughly for GMP inspections.
The material presented was prepared using data relevant to 30.09.2023. In case of new or additional data the article can be updated.
The Federal State Budgetary Institution “The All-Russian State Center for Quality and Standardization of Veterinary Drugs and Feed”, FSBI “VGNKI” is an institution authorized to conduct inspections of manufacturers of medicinal products for veterinary use manufactured outside the Russian Federation for compliance with the GMP rules requirements
The Center for Strategic Research Foundation, CSR Foundation or CSR is a Russian non-profit organization established in 1999 with the aim of developing and conducting strategic research on socio-economic topics
The Association of Veterinary Pharmaceutical Manufacturers AVPHARM is an association representing the interests of leading international pharmaceutical companies in the Russian Federation – manufacturers of medicinal products for veterinary use
REFERENCES:
- Электронный фонд правовых и нормативно-технических документов / Федеральный закон от 02.07.2021 г. № 317-ФЗ «О внесении изменений в Федеральный закон «Об обращении лекарственных средств». URL: https://docs.cntd.ru/document/607142404 (дата обращения 09.2023)
- Россельхознадзор / Деятельность / Госуслуги / Выдача заключения о соответствии производителя (иностранного производителя) лекарственных средств для ветеринарного применения требованиям правил надлежащей производственной практики / Дополнительная информация для заявителя / Государственный реестр заключений о соответствии производителя (иностранного производителя) лекарственных средств для ветеринарного применения требованиям правил надлежащей производственной практики. URL: https://fsvps.gov.ru/files/gosudarstvennyj-reestr-zakljuchenij-o-sootvetstvii-proizvoditelja-inostrannogo-proizvoditelja-lekarstvennyh-sredstv-dlja-veterinarnogo-primenenija-trebovanijam-pravil-nadlezhashhej-proizvodstvennoj-pr/ (дата обращения 30.09.2023)
- ФГБУ «ВГНКИ» / Пресс-центр / Новости / ФГБУ «ВГНКИ» представило результаты GMP-инспекций иностранных производителей лекарственных средств для ветеринарного применения за 2022 год. URL: https://www.vgnki.ru/fgbu-vgnki-predstavilo-rezultaty-gmp-inspekcij-inostrannyh-proizvoditelej-lekarstvennyh-sredstv-dlya-veterinarnogo-primeneniya-za-2022-god.html (дата обращения 30.09.2023)
- ФГБУ «ВГНКИ» / Структура / Отдел инспекции производства на соответствие требованиям надлежащей производственной практики / График проведения инспектирования иностранных производителей на соответствие требованиям надлежащей производственной практики. URL: https://www.vgnki.ru/assets/files/grafik_inspekcii/grafik-na-sajt-19092023.pdf (дата обращения 30.09.2023)
- ГК ВИК / Пресс-центр / Российские производители за два года закроют основные потребности по ветеринарным препаратам. URL: https://vicgroup.ru/press-center/rossiyskie-proizvoditeli-za-dva-goda-zakroyut-osnovnye-potrebnosti-po-veterinarnym-preparatam/ (дата обращения 30.09.2023)
- ФГБУ «ВГНКИ» / Пресс-центр / Новости / Анонс: на базе ФГБУ «ВГНКИ» пройдет обучение по порядку аттестации лица, уполномоченного держателем или владельцем регистрационного удостоверения лекарственного препарата. URL: https://www.vgnki.ru/anons-na-baze-fgbu-vgnki-projdet-obuchenie-po-poryadku-attestacii-lica-upolnomochennogo-derzhatelem-ili-vladelcem-registracionnogo-udostovereniya-lekarstvennogo-preparatahtml (дата обращения 30.09.2023)
- Официальный интернет-портал правовой информации / Приказ Министерства сельского хозяйства Российской Федерации от 10.11.2022 № 795 «Об утверждении Порядка аттестации лица, уполномоченного держателем или владельцем регистрационного удостоверения лекарственного препарата для ветеринарного применения». URL: http://publication.pravo.gov.ru/Document/View/0001202211290041 (дата обращения 30.09.2023)
- ФГБУ «ВГНКИ» / Пресс-центр / Новости / Анонс: в ФГБУ «ВГНКИ» пройдет семинар, посвященный правилам надлежащей дистрибьюторской практики лекарственных препаратов для ветеринарного применения (GDP). URL: https://www.vgnki.ru/anons-v-fgbu-vgnki-projdet-seminar-posvyashhennyj-pravilam-nadlezhashhej-distribyutorskoj-praktiki-lekarstvennyh-preparatov-dlya-veterinarnogo-primeneniya-gdphtml (дата обращения 30.09.2023)
- Официальный интернет-портал правовой информации / Приказ Министерства сельского хозяйства Российской Федерации от 29.03.2023 № 313 «Об утверждении Правил надлежащей дистрибьюторской практики лекарственных препаратов для ветеринарного применения». URL: http://publication.pravo.gov.ru/document/0001202306010055 (дата обращения 09.2023)
- ЦСР / События / Ввод в гражданский оборот ветпрепаратов: эффекты обновленного требования о сертификации GMP – исследование WCH. URL: https://www.csr.ru/ru/events/vvod-v-grazhdanskiy-oborot-vetpreparatov-effekty-obnovlennogo-trebovaniya-o-sertifikatsii-gmp-issledovanie-tssr/ (дата обращения 30.09.2023)
- АВФАРМ / Новости АВФАРМ / ООО «Эланко Рус» выходит из состава АВФАРМ. URL: https://avpharm.ru/news/elanco_leaves/ (дата обращения 30.09.2023)
- SinoPharmtech / Новости / 2-й китайско-российский форум по биофармацевтическим технологиям и деловому сотрудничеству / Программа форума. URL: https://sinopharmtech.com/ru/predvaritelnaja-programma-2-go-kitajsko-rossijskogo-foruma/ (дата обращения 30.09.2023)
- Ветеринария и жизнь / Зообизнес / В Китае вводят новые правила GMP для производителей ветпрепаратов. URL: https://vetandlife.ru/sobytiya/v-kitae-vvodyat-novye-pravila-gmp-dlya-proizvoditelej-vetpreparatov/ (дата обращения 30.09.2023)
- VIII Всероссийская GMP-конференция. Вопросы регулирования обращения лекарственных средств в условиях многополярного мира обсудили на GMP-конференции 2023. URL: https://gosgmp.ru/2023/09/28/voprosy-regulirovaniya-obrashheniya-lekarstvennyh-sredstv-v-usloviyah-mnogopolyarnogo-mira-obsudili-na-gmp-konferenczii-2023/ (дата обращения 09.2023)
- Фармстратегия / Открытые семинары и вебинары / Актуальные вопросы инспектирования производства стерильных лекарственных средств. Взгляд инспектора. URL: https://www.goodpractices.ru/upload/iblock/91d/cz0lzcndwu5yfpljcw3acoiaxwi2il13/231003_Anons_Inspektirovanie-pr_va-SLS_NF.pdf (дата обращения 30.09.2023)
- Евразийский экономический союз / Решение Совета Евразийской экономической комиссии от 03.11.2016 г. № 77 «Об утверждении Правил надлежащей производственной практики Евразийского экономического союза». URL: https://docs.eaeunion.org/docs/ru-ru/01411921/cncd_21112016_77 (дата обращения 30.09.2023)
- Евразийская Академия надлежащих практик / Образовательная деятельность Образовательные программы (план) / ПК «Подготовка аудиторов производства лекарственных средств». URL: https://gxp-academy.org/education/courses/podgotovka-auditorov-proizvodstva-lekarstvennykh-sredstv-23-2/ (дата обращения 30.09.2023)
- SCM Pharm / Мероприятия / V Международная конференция Логистика лекарственных средств. URL: https://scmpharm.ru/events/5-offline-conf/#terms (дата обращения 30.09.2023)
- Евразийская конференция по валидации. URL: https://validconf.ru/ (дата обращения 30.09.2023)
- Евразийский экономический союз / Решение Совета Евразийской экономической комиссии от 21.01.2022 № 1 «О Правилах регулирования обращения ветеринарных лекарственных средств на таможенной территории Евразийского экономического союза. URL: https://docs.eaeunion.org/Pages/DisplayDocument.aspx?s=%7Be1f13d1d-5914-465c-835f-2aa3762eddda%7D&w=9260b414-defe-45cc-88a3-eb5c73238076&l=%7B8a412e96-924f-4b3c-8321-0d5e767e5f91%7D&EntityID=32004 (дата обращения 30.09.2023)
- ФГБУ «ВГНКИ» / Пресс-центр / Новости / ФГБУ «ВГНКИ» провело обучение для сотрудников казахского Национального референтного центра по ветеринарии. URL: https://www.vgnki.ru/fgbu-vgnki-provelo-obuchenie-dlya-sotrudnikov-kazahskogo-nacionalnogo-referentnogo-centra-po-veterinarii.html (дата обращения 30.09.2023)
