
The quarterly review provides information on the inspection of manufacturers of veterinary medicines for compliance with the requirements of the Good Manufacturing Practice (GMP) which is carried out by specialists of the Inspection Body of the Federal State Budgetary Institution “The All-Russian State Center for Quality and Standardization of Veterinary Drugs and Feed” (FSBI “VGNKI”).
Inspection results
Foreign manufacturers
According to the register of conclusions, published on the Rosselkhoznadzor website 16.06.2025 [1], in the second quarter of this year, 2 conclusions on compliance with the GMP Rules requirements were issued. These conclusions were made for sites located in Korea and France.
In accordance with the inspection schedule published on the VGNKI website 30.06.2025 [2], 6 inspections of manufacturers of veterinary medicinal products are planned for the third quarter of 2025, which sites located in Vietnam, Israel, Spain, China, New Zealand and Switzerland.
According to the register [1], today foreign manufacturers have 60 valid GMP-conclusions.
In May, the Rosselkhoznadzor website published information that, based on the results of a random inspection of veterinary medicines in circulation and due to the lack of a system for monitoring veterinary medicines by the competent Israeli authority, the agency organized an unscheduled inspection of one of the Israeli manufacturers. Due to the identification during the inspection of a number of violations indicating a systemic failure in quality management, Rosselkhoznadzor suspended the GMP-conclusions and prohibited the import of vaccines produced by the Israeli enterprise until all identified non-compliances are eliminated in accordance with GMP requirements [3, 4].
The largest number of valid conclusions are held by Spanish sites (9, which is 15%).
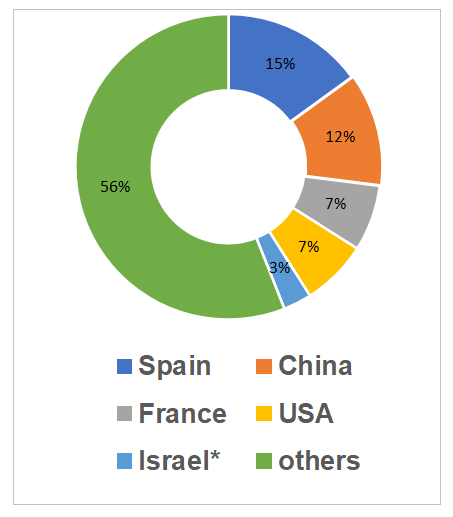
*2 conclusions suspended
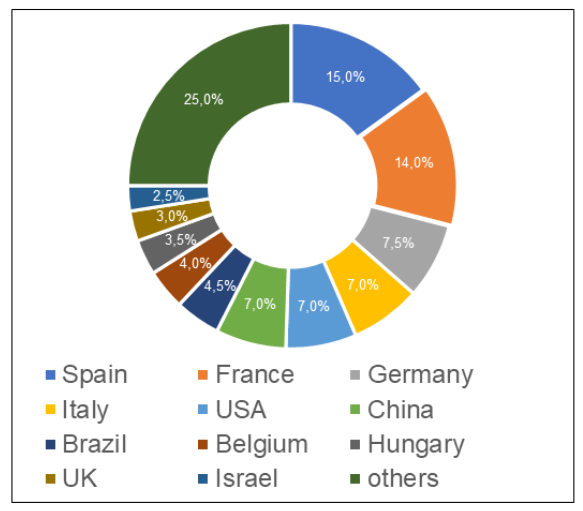
At the same time, the largest number of Russian inspections, based on the results of which, over all the years of inspection, decisions were made to issue/refuse to issue a GMP-conclusion, were carried out at manufacturing sites also located in Spain (39, which is 15%).
According to a study of the pharmaceutical sector in Spain, carried out by the consultancy firm ManageArt for the Farmaindustria association, the pharmaceutical industry in Spain has 106 plants producing medicines for human use. If we add the plants producing active ingredients (46) and veterinary medicines (22), the total number of pharmaceutical plants in Spain is 174. According to Pedro Luis Sánchez, Director of the research department at Farmaindustria, in recent years, medicines have been among the top five most exported products in Spain. The highest concentration of pharmaceutical plants (including those producing veterinary medicines) is found in the north-east of the country – in Catalonia, where 79 of the 174 plants are located [5].
In May, within the framework of the 92nd annual General Session of the World Assembly of Delegates of the WOAH, Rosselkhoznadzor held a meeting with the Head of the General Directorate for Sanitation of Agri-Food Production and Animal Welfare of the Ministry of Agriculture, Fisheries and Food of Spain, Valentin Almanza de Lara, on the issue of supplies of safe medicinal products for veterinary use to Russia. The Russian agency asked to provide information on state veterinary supervision during the import of raw materials to Spain, including SPF eggs, to produce the specified products and a list of the studies carried out [4].
According to the industry publication Agroexpert, Rosselkhoznadzor may restrict the import of vaccines for veterinary use from two Spanish companies into Russia, since the agency has not received an official response about the suppliers of raw materials used in production, including SPF eggs [6].
The Russian agency’s high concern about this issue is understandable. Specific pathogen-free (SPF) eggs are an important raw material to produce vaccines and diagnostic medicines, as well as for research purposes in the field of veterinary and medical virology. GMP in the context of SPF eggs means compliance with strict rules and procedures at all stages of production to guarantee the absence of contamination and high quality of the product. In accordance with p. 34. of Annex 5 to the GMP Rules ” Manufacture of Immunological Veterinary Medicinal Products” [7, 8], specifications for starting materials should include details of the supplier, the method of manufacture, the geographical origin and the animal species from which the materials are derived; the controls to be applied to starting materials must be included. P. 36 states that special attention should be paid to a knowledge of the supplier’s quality assurance system in assessing the suitability of a source and the extent of quality control testing required.
Dialogue with an expert

Lidia Lianiuka Lenivko, consultant and trainer in preparation for GMP-inspections, founder of the consulting company RuGMP, specialist in relations between the pharmaceutical industry of Spain and Latin America with Russia and the EAEU countries answered several questions regarding GMP-inspections and linguistic support for inspections in Spain.
Lidia, as I understand from the analysis of ManageArt, almost 30% of the production of high-tech goods in Spain is provided by the pharmaceutical industry, and the development, production and export of medicines are supported by government programs. And how are things going on Spanish sites with ensuring GMP requirements? Are there specialized institutes for training specialists in the field of pharmaceutical production, quality control and assurance? Are there any advanced training programs, are there meetings of industry representatives with regulators?
Of course, Konstantin. The high level of the pharmaceutical industry in Spain would be impossible without a powerful, multi-level education system and strict regulatory control.
The system here is built very logically. After receiving higher education, many specialists deepen their knowledge in master’s programs focused specifically on the pharmaceutical industry. For example, I completed such a master’s program in pharmaceutical technology and drug registration at the Higher School of the Pharmaceutical Industry CESIF.
In addition, there are many short-term courses on specific topics. The companies themselves are also actively involved in training, organizing annual advanced training courses both on their own and with the involvement of external training companies. We are also regularly invited to conduct training on preparation for GMP-inspections.
An important role is also played by industry associations, such as the Spanish Association of the Pharmaceutical Industry (AEFI), of which I am a member. They organize both courses and meetings with the Spanish regulator AEMPS. Although, as often happens, there is a dialogue between the industry and the regulator, the industry would always like to see it even more active.
So, yes, it is safe to say that the level of compliance with GMP requirements at Spanish sites is traditionally high.
In one of articles, you mentioned that an interpreter at GMP-inspections should have prior experience working at such inspections, and a plus would be relevant education or courses, or work at pharmaceutical enterprises [9]. How do you improve your qualifications in this area, do you undergo any training on specific GMP topics?
This is a very important question that directly concerns the quality of our work and guarantees for the client. The fact is that RuGMP is a highly specialized company. We work exclusively in the field of GMP-inspections and audits for manufacturers of medicinal products for human use, veterinary medicines and medical devices. We do not translate in general areas such as tourism or marketing. It is this narrow specialization that obliges us to impose strict requirements on the qualifications of translators in our team.
Our minimum standards are higher education, at least 15 years of experience in interpreting and participation in at least 15 relevant audits or inspections. Of course, a specialized education is a huge plus: pharmacy, chemistry, biology. I graduated from the biology faculty and from personal experience I understand how important this knowledge is for a deep understanding of the essence of the matter, and not just a simple translation of words. Therefore, we provide clients with guarantees that the specialist working on their site fully meets these requirements.
But even such a strict selection is not a reason to stop. The pharmaceutical industry is very dynamic, and it itself requires constant development from us. This includes training in pharmaceutical translation courses offered by translation schools and associations, and our internal training. At the same time, our goal is not to make a quality specialist out of a interpreter, but to give him/her an understanding of the essence of the processes. He/she must clearly distinguish, for example, between validation and qualification, so that the slightest misunderstanding does not lead the dialogue in the wrong direction.
And yet, I consider the qualification acquired “in the field” to be the key factor, the basis of our expertise. Each inspection is the most valuable and relevant training. This is why we approach preparation so seriously: we request materials from customers, based on which our team develops multilingual glossaries and, if necessary, holds briefings for interpreters. After all, it is physical presence at the sites that allows us to learn first-hand about new trends and inspectors’ expectations. This practical experience cannot be obtained from the Internet, and this experience is the foundation of our expertise.
Professional interpreters who often participate in GMP-inspections tell of funny cases related to the translation of certain terms or abbreviations. For example, Alexander Podarevsky cited cases when pest control was translated as “pesticide control”, and the term “nutrient medium” sounded like “soil” [10]. Have you had similar cases in your practice and is it possible to avoid such confusion?
You have touched upon an interesting topic. I would like to point out right away that the incidents with the translation of basic terms, as in the examples you have given, are rather a reality of the past, approximately 2016-2019. At that time, many interpreters really had less experience in pharmaceutical technology. I can remember how I heard “platters” instead of “Petri dishes”. Today, the level of expertise in the industry has increased, and experienced interpreters no longer make such mistakes.
However, this does not mean that funny cases have disappeared. They have simply become more subtle and complex. Now they are concerned not basic terminology, but internal slang and specific abbreviations of the enterprise. These cases perfectly illustrate why context is more important than literal meaning in our work. I recall a recent Russian GMP-inspection. During a tour of the production, technical personnel began to discuss the “wifi” network scheme. Our interpreters were confused: what does wireless Internet have to do with the manufacture of sterile medicines? Instead of guessing, they did what professionals should do: they stopped and asked for context. The quality manager laughed. It turned out that at the plant they abbreviated WFI (Water for Injection) this way, and it was so common for them that no one thought about the phonetic similarity.
Such embarrassments can and should be avoided. That is why our working method is based on proactive preparation. We do not wait for a list of terms from the client. We request process flow diagrams, process charts and other documentation. Based on these materials, our team independently prepares a contextual glossary. We pay special attention to deciphering internal abbreviations that are unique to each enterprise. This approach allows us to guarantee accuracy and avoid such “surprises”.
As an interpreter, you have participated in many inspections. Foreign manufacturers of veterinary medicines will soon begin to undergo joint GM- inspections under the Rules for Conducting Pharmaceutical Inspections, which are an Appendix to the “Rules for Regulating the Circulation of Veterinary Medicines in the Customs Territory of the EAEU” [11]. Is there a significant difference between inspections conducted by the inspectorate of only one country and joint inspections? What should be noted?
The main difference between joint inspections is, of course, the increased complexity of the process. When an inspection is carried out by one country, the manufacturer deals with common expectations and inspection “school”. In the case of joint inspections of the EAEU, despite the general rules, the group includes inspectors from different member states.
Our experience shows that each inspector can have his own focus, his own “favorite” topics based on his personal experience and the practice of the regulator of his country. This adds a new level of internal dynamics to the process. Therefore, we always advise sites to be prepared for the fact that questions on the same topic may come from different angles. In such a situation, not only impeccable technical preparation is important, but also a certain diplomatic flexibility in communicating with the team of inspectors.
About five years ago, you held a webinar on the topic “What can be done in the week before a GMP inspection or audit?”, where you talked about how a site can identify and correct up to 20% of potential non-conformities during the preparation stage. Has your opinion changed over the years? Are there any new tips for manufacturers preparing for inspections?
My main opinion has not changed: preparation is the key to everything. The week before the inspection is still critically important for “polishing” and eliminating minor flaws: checking the relevance of document versions, the presence of all signatures, the correctness of corrections, and so on.
However, our approach has really evolved over the years. If earlier the emphasis was on having time to “fix”, today we focus on “tuning” – tuning the team to work harmoniously and confidently. Therefore, my main advice is the following: devote this last week not to a panic search for non-compliances, but to working with the team. Conduct internal rehearsals in the format of mock inspections with questions and answers.
The main goal is to ensure that each employee not only knows his procedure but also understands why the process is structured this way and can calmly and confidently explain it. To do this, it is important to:
-
- clearly define the roles of personnel during the inspection: who accompanies, who prepares the documentation, who coordinates the process;
- help each key employee prepare for their interview: think through the topics they will cover in advance and plan for their answers.
It is these aspects of teamwork and communication that we place special emphasis on in our training in preparation for an inspection. As a result, we see that in a week it is not so much the documentation that can be improved, but the “mentality” of the team: helping it move from a state of stress to a state of calm confidence. And it is this calmness and coherence that inspectors read first and foremost.
Russian manufacturers
According to the register on the Rosselkhoznadzor website [1], in the second quarter of the current year, a GMP-conclusion was issued to Goros21.ru manufacturing site. To date, domestic manufacturers have 30 valid conclusions.
Since March 2022, the Decree of the Government of the Russian Federation dated 12.03.2022 No. 353 “On the Specifics of Licensing Activities in the Russian Federation” [12] has been in force. In accordance with paragraph 1 of Appendix No.20 to this Decree, for veterinary medicinal products manufactured in the Russian Federation for the purpose of import substitution, including original medicinal products, an accelerated state registration procedure is established, not exceeding 60 working days, due to the waiver of the quality examination of the veterinary medicinal product, subject to the submission of a conclusion on the compliance of the manufacturing site with the requirements of the Rules of Good Manufacturing Practice (with the exception of live vaccines and immunobiological products against particularly dangerous animal diseases specified in the list of contagious, including particularly dangerous, animal diseases for which restrictive measures (quarantine) may be established, approved by the Ministry of Agriculture of the Russian Federation).
Past events and activities
In the second quarter of this year, several events and activities took place that might be of interest to manufacturers preparing for inspection.
In April, the XIV International Pharmaceutical Forum PharmPRO 2025 was held in Moscow. Practical case sessions on production and quality, promotion and sales, and personnel management gave each participant the opportunity to expand their professional arsenal with new solutions [13].
Maria Timchenko, Head of the Finished Products Coordination Department at STPF Polisan company, took part in the session “From process quality to product quality and production efficiency” with the topic “Warehouse with a Ministry of Industry and Trade License. Why is this so important for organizing storage processes?” She presented the following three options.
- Adding a warehouse with a pharmaceutical license to the manufacturer’s license, while:
- the warehouse should have a quality system, climate monitoring, warehouse mapping, rodent/insect control systems, rejected/quarantine zones, etc.
- there should be a Qualified person;
- storage of raw materials, materials, finished products (at any stage relative to release) is possible;
- the manufacturing company will need to actively participate in the life of the warehouse quality system (audits, quality agreement, control by the manufacturer).
- Concluding a storage agreement with a company that has a pharmaceutical license, while:
- storage in such a warehouse is possible only in the event of transfer of ownership to the second company (including transfer under the monitoring the movement of medicines);
- restrictions: storage of finished products only and only after release and transfer of ownership.
- Construction of your own warehouse.

In the same month, the 23rd International Exhibition “Analitika Expo 2025” was held in Moscow. On April 23, within the framework of the exhibition, the “Forum of specialists on the quality and registration of medicinal products” was held, the partner of which was the GxP Training Center [14].
In his report “Risk management in the framework of pharmaceutical quality audits” Dmitry Chukhaev, Validation engineer at Astrapharm Group of Companies, listed the main regulatory documents in this area:
- The Federal Law dated 12.04.2010 No. 61-FZ “On the Circulation of Medicines” [15];
- Decision of the EEC Council dated 03.11.2016 No. 77 “On Approval of the Rules of Good Manufacturing Practice of the Eurasian Economic Union” (with subsequent amendments) [7];
- PIC/S PI 037-1 “A Recommended Model for Risk-based Inspection Planning in the GMP Environment” [16].
The speaker focused in more detail on the last of the listed documents, which sets out a simple and flexible Quality Risk Management tool that may be used by Inspectorates and auditors when planning the frequency and scope of GMP inspection/audit.

Upcoming events and activities
On September 16-18, the X All-Russian GMP Conference will take place in Moscow. The organizer of the conference is the Ministry of Industry and Trade of Russia together with the FSI “SID & GP”. The All-Russian GMP Conference has been held since 2016 and annually brings together leading international and Russian experts in the pharmaceutical industry, representatives of government authorities, managers and specialists of pharmaceutical production, representatives of professional associations and communities, experts in the field of quality management systems and GMP [17].
Manufacturers are encouraged to take an active part in GM(D)P-related events and activities and prepare more thoroughly for GMP inspections.
The material presented was prepared using data relevant to 10.07.2025. In case of new or additional data the article can be updated.
The Federal State Budgetary Institution “The All-Russian State Center for Quality and Standardization of Veterinary Drugs and Feed”, FSBI “VGNKI” is an institution authorized to conduct inspections of manufacturers of medicinal products for veterinary use manufactured outside the Russian Federation for compliance with the GMP Rules requirements
The World Organisation for Animal Health, WOAH is an intergovernmental organization responsible for improving animal health worldwide, coordinating, promoting and combating animal diseases, food safety and animal welfare
References:
- Россельхознадзор / Деятельность / Госуслуги / Выдача заключения о соответствии производителя (иностранного производителя) лекарственных средств для ветеринарного применения требованиям правил надлежащей производственной практики / Дополнительная информация для заявителя / Государственный реестр заключений о соответствии производителя (иностранного производителя) лекарственных средств для ветеринарного применения требованиям правил надлежащей производственной практики. URL: https://fsvps.gov.ru/ru/gosuslugi/zaklyuchenie-o-sootvetstvii (дата обращения 10.07.2025)
- ФГБУ «ВГНКИ» / Структура / Отдел инспекции производства на соответствие требованиям надлежащей производственной практики / График проведения инспектирования иностранных производителей на соответствие требованиям надлежащей производственной практики. URL: https://www.vgnki.ru/assets/2024/files/grafik-na-sajt-30062025.pdf (дата обращения 10.07.2025)
- Россельхознадзор / Новости/ Россельхознадзор запретил ввоз в Россию вакцин израильского производителя Абик Биолоджикал Лабораторис. URL: https://fsvps.gov.ru/news/rosselhoznadzor-zapretil-vvoz-v-rossiju-vakcin-izrailskogo-proizvoditelja-abik-biolodzhikal-laboratoris/ (дата обращения 10.07.2025)
- Россельхознадзор / Новости / Россельхознадзор на полях Генсессии ВОЗЖ провел переговоры с представителями ветеринарных служб Испании и Израиля. URL: https://fsvps.gov.ru/news/rosselhoznadzor-na-poljah-gensessii-vozzh-provel-peregovory-s-predstaviteljami-veterinarnyh-sluzhb-ispanii-i-izrailja/ (дата обращения 10.07.2025)
- Farmaindustria / La industria farmacéutica alcanza las 174 plantas de producción de medicamentos en hasta 13 comunidades autónomas españolas. URL: https://www.farmaindustria.es/web/prensa/notas-de-prensa/2024/07/10/espana-una-potencia-en-la-fabricacion-de-medicamentos/ (дата обращения 10.07.2025)
- Агроэксперт / Животные и птица / РФ заподозрила испанских производителей ветеринарных вакцин в использовании зараженного сырья. URL: https://agroexpert.press/zhivotnye-ryba-pticza/rf-zapodozrila-ispanskih-proizvoditelej-veterinarnyh-vakczin-v-ispolzovanii-zarazhennogo-syrya/ (дата обращения 10.07.2025)
- Евразийский экономический союз / Решение Совета Евразийской экономической комиссии от 03.11.2016 г. № 77 «Об утверждении Правил надлежащей производственной практики Евразийского экономического союза». URL: https://docs.eaeunion.org/docs/ru-ru/01411921/cncd_21112016_77 (дата обращения 10.07.2025)
- The rules governing medicinal products in the European Union – Volume 4 – EU Guidelines for Good Manufacturing Practice for Medicinal Products for Human and Veterinary Use / Annex 5: Manufacture of Immunological Veterinary Medicinal Products. URL: https://health.ec.europa.eu/document/download/c14c2480-2473-4866-bf2b-d0b7ac61bc27_en?filename=anx05en200408_en.pdf (дата обращения 10.07.2025)
- GxP News / Анонсы / Новости / Готовь сани летом, или Организация подготовки и прохождения GxP инспекций. URL: https://gxpnews.net/2019/03/gotov-sani-letom-ili-organizaciya-podgotovki-i-proxozhdeniya-gxp-inspekcij/ (дата обращения 10.07.2025)
- ФармПром.РФ / Анонсы / Вышел второй номер отраслевого журнала «ФАРМПРОМ» за 2023 год в формате онлайн. URL: https://pharmprom.ru/vyshel-vtoroj-nomer-otraslevogo-zhurnala-farmprom-za-2023-god-v-formate-onlajn/ (дата обращения 10.07.2025)
- Евразийский экономический союз / Решение Совета Евразийской экономической комиссии от 21.01.2022 № 1 «О Правилах регулирования обращения ветеринарных лекарственных средств на таможенной территории Евразийского экономического союза. URL: https://docs.eaeunion.org/Pages/DisplayDocument.aspx?s=%7Be1f13d1d-5914-465c-835f-2aa3762eddda%7D&w=9260b414-defe-45cc-88a3-eb5c73238076&l=%7B8a412e96-924f-4b3c-8321-0d5e767e5f91%7D&EntityID=32004 (дата обращения 10.07.2025)
- КонсультантПлюс / Постановление Правительства РФ от 12.03.2022 N 353 (ред. от 12.06.2025) “Об особенностях разрешительной деятельности в Российской Федерации”. URL: https://www.consultant.ru/document/cons_doc_LAW_411447/ (дата обращения 10.07.2025)
- PharmPRO / XIV фармацевтический форум PharmPRO. URL: https://events.pharmpro.pro/forum-2025 (дата обращения 10.07.2025)
- Аналитика Экспо / Деловая программа / Форум специалистов по качеству и регистрации лекарственных средств. URL: https://analitikaexpo.com/ru/agenda/bp_25/SmartLab/Day1-forum/ (дата обращения 10.07.2025)
- КонсультантПлюс / Федеральный закон “Об обращении лекарственных средств” от 12.04.2010 N 61-ФЗ (последняя редакция). URL: https://www.consultant.ru/document/cons_doc_LAW_99350/ (дата обращения 10.07.2025)
- PIC/S / Recommendation PI 037-1 A Recommended Model for Risk-based Inspection Planning in the GMP Environment. URL: https://picscheme.org/docview/3439 (дата обращения 10.07.2025)
- X Всероссийская GMP-конференция. URL: http://gosgmp.ru/ (дата обращения 10.07.2025)

