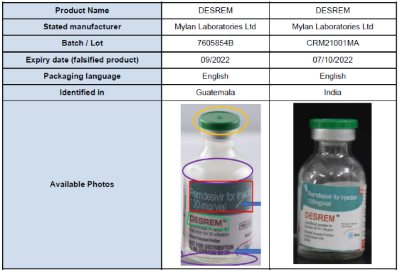
The genuine manufacturer of DESREM, Mylan Laboratories Ltd, has confirmed that the products identified in this Alert are falsified. Falsified DESREM (Remdesivir) identified in the WHO Regions of the Americas and South-East Asia.
Laboratory analysis of these falsified products, conducted by the genuine manufacturer, established that they do not contain any of the stated active pharmaceutical ingredient (remdesivir). The vials of these falsified products may be smaller than genuine DESREM and the labels have multiple spelling errors and use the wrong font styles and colours. Although the identified batch numbers are genuine, the expiry dates listed below are falsified.
The products identified in this Alert are falsified on the basis that they deliberately/fraudulently misrepresent their identity, composition, and source.
Falsified remdesivir products pose a risk to global public health and hamper efforts to treat patients with COVID-19. Such falsified products place an additional burden on vulnerable populations and health systems.
The risk to patient health from the falsified product identified in this Alert may include a delay in receiving safe and effective treatment.
It is important to detect and remove these falsified products from circulation to prevent harm to patients, WHO states.
