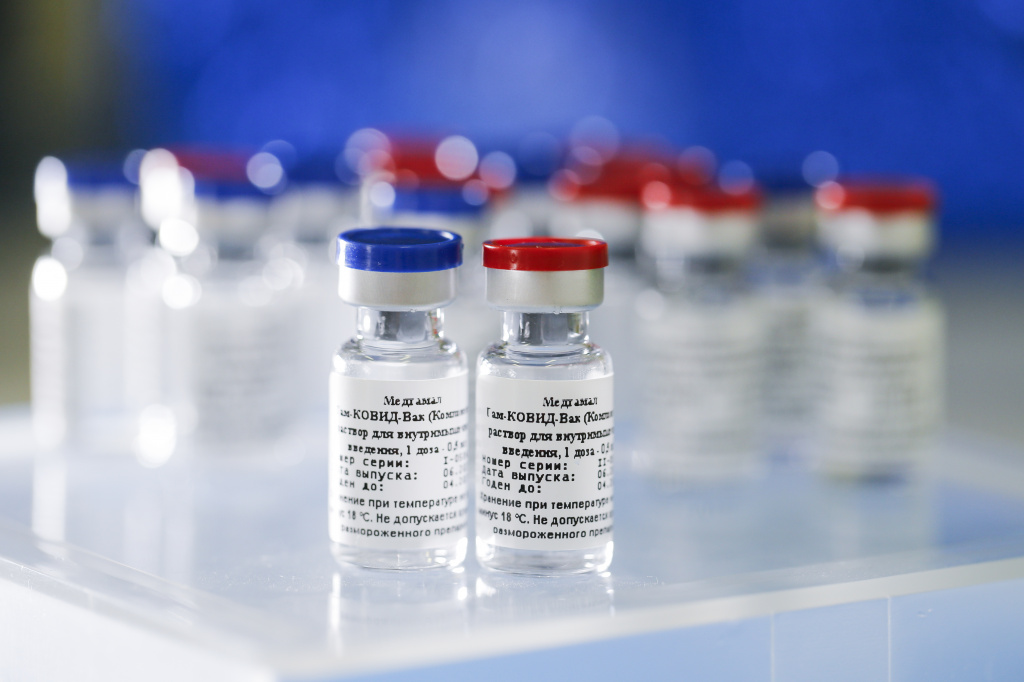
Sputnik V COVID-19 vaccine
Sputnik V (Russian: Спутник V) is an adenovirus viral vector vaccine for COVID-19 developed by the Gamaleya Research Institute of Epidemiology and Microbiology. It was registered on 11 August 2020 by the Russian Ministry of Health as Gam-COVID-Vac (Russian: Гам-КОВИД-Вак). The ‘V’ in its name is the letter and not the Roman numeral.
Gam-COVID-Vac was initially approved for distribution in Russia and then in 59 other countries (as of April 2021) on the preliminary results of Phase I–II studies eventually published on 4 September 2020. Approval in early August of Gam-COVID-Vac was met with media criticism in mass media and discussions in the scientific community as to whether approval was justified in the absence of robust scientific research confirming safety and efficacy. On 2 February 2021, an interim analysis from the trial was published in The Lancet, indicating 91.6% efficacy without unusual side effects.
Emergency mass-distribution of the vaccine began in December 2020 in countries including Russia, Argentina, Belarus, Hungary, Serbia and the United Arab Emirates. By February 2021 over a billion doses of the vaccine had been ordered for immediate distribution worldwide.
Source: Wikipedia
Latest posts by tag Sputnik V
- India’s production, Russia’s export of Sputnik vaccine at risk due to Ukraine crisis
- Markus Soeder: Sputnik V vaccine project in Bavaria is closed
- Vaccine against COVID Sputnik V granted full permanent approval in Russia
- Russia and China are working on mutual recognition of COVID-19 vaccines
- Argentina displays interest in nasal form of Russia’s Sputnik V vaccine
[rev_slider alias="ransom-950x120-en"][/rev_slider]