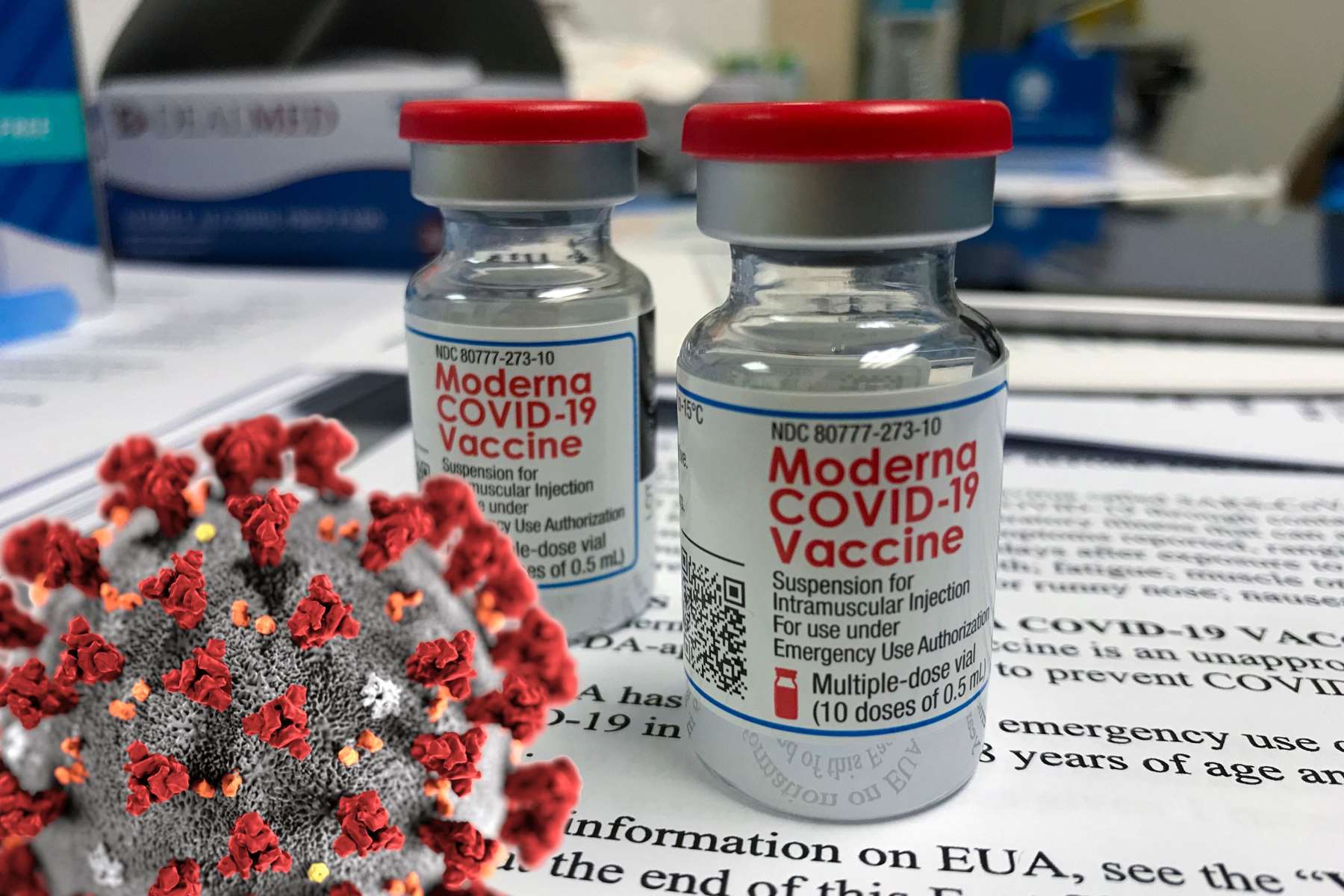
Moderna COVID-19 Vaccine
The Moderna COVID‑19 vaccine, codenamed mRNA-1273, is a COVID-19 vaccine developed by Moderna, the United States National Institute of Allergy and Infectious Diseases (NIAID) and the Biomedical Advanced Research and Development Authority (BARDA). It is used in people aged 18 years and older to provide protection against infection by the SARS-CoV-2 virus, which causes COVID-19. It is designed to be administered as two 0.5 mL doses given by intramuscular injection at an interval of four weeks apart.
It is an RNA vaccine composed of nucleoside-modified mRNA (modRNA) encoding a spike protein of SARS-CoV-2, which is encapsulated in lipid nanoparticles.
The Moderna COVID‑19 vaccine is authorized for use at some level in 53 countries including the United States, Canada, the European Union, the United Kingdom, Israel, and Singapore.
On 15 March 2021, Moderna’s second COVID‑19 vaccine (mRNA-1283) started phase I clinical trials.
Medical uses
The Moderna COVID‑19 vaccine is used to provide protection against infection by the SARS-CoV-2 virus in order to prevent COVID‑19.
Efficacy
Evidence of vaccine efficacy starts about two weeks after the first dose. High efficacy is achieved with full immunization, two weeks after the second dose, and was evaluated at 94.1%: at the end of the vaccine study that led to emergency authorization in the USA, there were eleven cases of COVID‑19 in the vaccine group (out of 15,181 people) versus 185 cases in the placebo group (15,170 people). Moreover, there were zero cases of severe COVID‑19 in the vaccine group, versus eleven in the placebo group. This efficacy has been described as “astonishing” and “borderline historic” for a respiratory virus vaccine, and it is similar to the efficacy of the Pfizer–BioNTech COVID-19 vaccine.
Standard
On 6 January 2021, the European Medicines Agency (EMA) recommended granting conditional marketing authorization and the recommendation was accepted by the European Commission the same day. On 6 June 2021, the EMA started evaluating an application to extend the use of the COVID‑19 Vaccine Moderna to include people aged 12 to 17.
On 12 January 2021, Swissmedic granted temporary authorization for the Moderna COVID-19 mRNA Vaccine in Switzerland.
On 1 April 2021, the Medicines and Healthcare products Regulatory Agency granted full marketing authorization in the United Kingdom.
The Moderna COVID‑19 vaccine is a vaccine for preventing coronavirus disease 2019 (COVID-19) in people aged 18 years and older.
COVID-19 Vaccine Moderna contains a molecule called messenger RNA (mRNA) with instructions for producing a protein from SARS-CoV-2, the virus that causes COVID-19. Vaccine does not contain the virus itself and cannot cause COVID-19.
Moderna COVID‑19 vaccine is given as two injections, usually into the muscle of the upper arm, 28 days apart. Arrangements for the supply of the vaccine will be the responsibility of national authorities.
Moderna COVID‑19 vaccine is now authorised across the EU. This follows the granting of a conditional marketing authorisation by the European Commission on 6 January 2021.
EMA’s human medicines committee (CHMP) has thoroughly assessed the data on the quality, safety and efficacy of the vaccine and recommended by consensus a formal conditional marketing authorisation be granted by the European Commission.
A very large clinical trial showed that COVID-19 Vaccine Moderna was effective at preventing COVID-19 in people from 18 years of age.
The trial involved around 30,000 people in total. Half received the vaccine and half were given dummy injections. People did not know whether they received the vaccine or the dummy injections. Efficacy was calculated in around 28,000 people from 18 to 94 years of age who had no sign of previous infection.
The trial showed a 94.1% reduction in the number of symptomatic COVID-19 cases in the people who received the vaccine (11 out of 14,134 vaccinated people got COVID-19 with symptoms) compared with people who received dummy injections (185 out of 14,073 people who received dummy injections got COVID-19 with symptoms). This means that the vaccine demonstrated a 94.1% efficacy in the trial.
The trial also showed 90.9% efficacy in participants at risk of severe COVID-19, including those with chronic lung disease, heart disease, obesity, liver disease, diabetes or HIV infection. The high efficacy was also maintained across genders, racial and ethnic groups.
Moderna COVID‑19 vaccine is given as two injections into the arm, 28 days apart. The most common side effects with COVID-19 Vaccine Moderna were usually mild or moderate and got better within a few days after vaccination. The most common side effects are pain and swelling at the injection site, tiredness, chills, fever, swollen or tender lymph nodes under the arm, headache, muscle and joint pain, nausea and vomiting.
[rev_slider alias="ransom-950x120-en"][/rev_slider]