
The quarterly review provides information on the inspection of manufacturers of veterinary medicines for compliance with the requirements of the Good Manufacturing Practice (GMP) which is carried out by specialists of the Inspection Body of the Federal State Budgetary Institution “The All-Russian State Center for Quality and Standardization of Veterinary Drugs and Feed” (FSBI “VGNKI”).
Inspection results
Foreign manufacturers
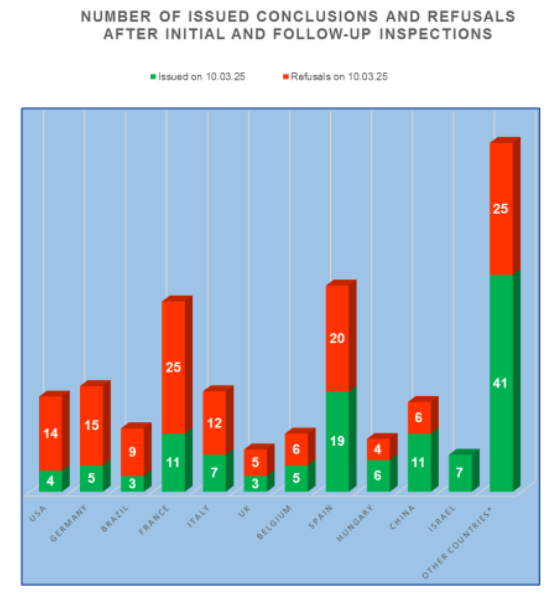
According to the register of conclusions, published on the Rosselkhoznadzor website 10.03.2025 [1], since the beginning of this year, 3 conclusions on compliance with the GMP Rules requirements were issued. These conclusions were made for sites located in located in in China, the Netherlands and Croatia.
An analysis of the results of inspections in those countries where in 2017-2025 the largest number of Russian inspections were carried out shows the following trends:
- the worst results are at the manufacturing sites located in the USA, Germany and Brazil (more than 75% of refusals to issue a conclusion);
- good results are at the manufacturing sites located in China, Hungary and Spain (no more than 51% of refusals to issue a conclusion);
- the best results are at the manufacturing sites located in in Slovenia, Israel and Portugal (no refusal to issue a conclusion).
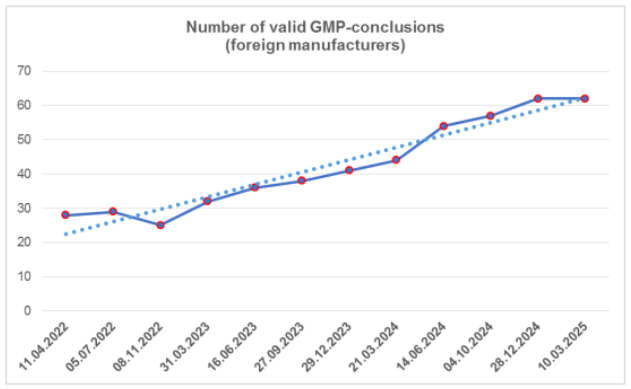
According to the register [1], today foreign manufacturers have 62 valid GMP-conclusions.
At the conference “PROZooRetail: Rules of the Game” held in March this year, Nikolay Bespalov, Development director of the analytical company RNC Pharma, gave a presentation on “The retail market of veterinary medicines in Russia. Results of 2024 and development prospects in 2025.” [2]. According to the data presented, the top 10 corporations in terms of sales in the retail market of veterinary medicines in Russia included such foreign manufacturers as Zoetis, Elanco, KRKA, MSD, Boehringer Ingelheim and Bioveta.
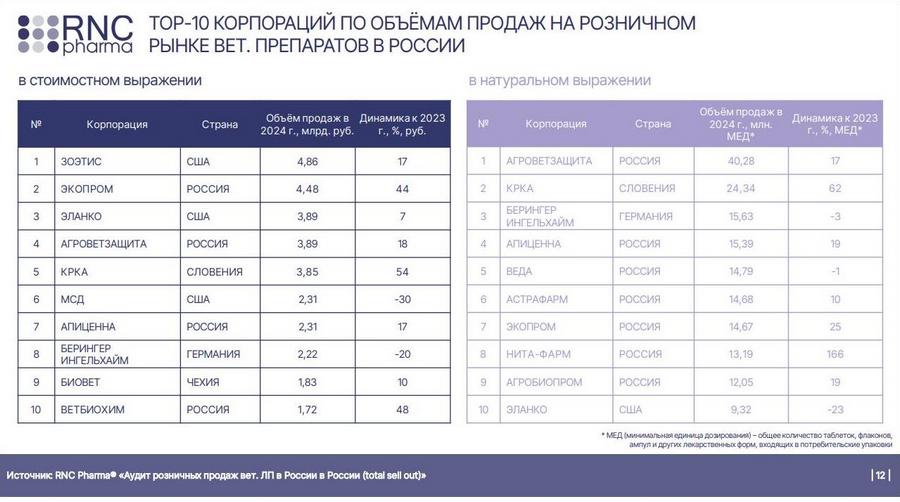
Today, manufacturing sites and CMOs of these foreign companies have a total of more than 40% valid GMP-conclusions.
At the annual board meeting of Rosselkhoznadzor, Deputy Prime Minister Dmitry Patrushev noted [3] that, starting in 2023, a mandatory GMP certificate [conclusion] is required for the introduction of medicines into circulation: “Conscientious market participants received such certificates on time and continue to interact with Russian buyers.”
In accordance with the inspection schedule published on the VGNKI website 14.03.2025 [4], 8 inspections of manufacturers of veterinary medicinal products are planned for the second quarter of 2025, which sites located in in Brazil, Hungary, India, Spain, China, Uruguay and France.
Russian manufacturers
According to the register on the Rosselkhoznadzor website [1], GMP-conclusions were received by the manufacturing sites of NITA-FARM and Biokhimik. To date, domestic manufacturers have 33 valid conclusions.
More and more companies manufacturing medicinal products for human use have begun to receive a conclusion from Rosselkhoznadzor on the compliance of the manufacturer of medicinal products for veterinary use with the GMP Rules requirements. For example, in 2023-2024, Biokhimik (a production complex of the PROMOMED Group) prepared for the start of production of veterinary medicinal products, including the validation of production lines and the release of pilot batches of four medicinal products for animals with full quality control. The received certificate [conclusion] allows PROMOMED to register veterinary medicines under a simplified procedure and bring their products to the market of the Russian Federation and the EAEU countries [5].
According to Deputy Prime Minister Dmitry Patrushev [3], the Government will allocate 5 billion rubles to the creation of Russian veterinary medicines by 2030: “The main thing for us is, of course, strengthening our own positions in this area. Within the framework of the new national project [“Technological Support for Food Security”], there is a separate direction for the creation of domestic veterinary medicines.”
Past events and activities
In the first quarter of this year, several events and activities took place that might be of interest to manufacturers preparing for inspection.
In February, the Russian Ministry of Agriculture prepared a draft decree of the Government of the Russian Federation “On determining the compliance of manufacturers of medicinal products with the requirements of the Good Manufacturing Practice Rules” [6]. This draft proposes to approve the Rules establishing the procedure for organizing and conducting inspections of manufacturers of medicinal products for compliance with the requirements of the GMP Rules, as well as issuing conclusions on the compliance of the manufacturer of medicinal products with the requirements of the GMP Rules. The draft stipulates that this decree shall enter into force on September 1, 2025 and be valid until September 1, 2031. It is planned to recognize as invalid the Decree of the Government of the Russian Federation dated 03.12.2015 No. 1314 [7], which currently regulates the said legal relations.
In the same month, a working meeting of experts was held at the Russian Union of Industrialists and Entrepreneurs (RSPP) to develop proposals for amendments to the “Rules for regulating the circulation of veterinary medicinal products in the customs territory of the EAEU”, approved by the Decision of the EEC Council of 21.01.2022 No. 1 [8]. Interested members of the RSPP Commission on the Agro-Industrial Complex and Food Security, representatives of industry unions and associations, leading agro-industrial complex companies and federal executive bodies were invited to participate in this event [9].

Many of the proposals presented to the experts related to the Rules for conducting pharmaceutical inspections, which are Annex 26 to these Rules, in particular:
- Bring paragraph 5 into line with the approved form of the EAEU GMP certificate in Annex 1: remove from the list of documents that are submitted to the authorized body to obtain a certificate, the list of veterinary medicinal products manufactured (planned for manufacturing) at the manufactured site, since the Certificate is issued for manufacturing operations and dosage forms.
- Supplement paragraph 6: within 20 working days from the date of receipt of the relevant notification, the applicant shall provide the missing documents, to provide the applicant with additional time to correct the discrepancies in the submitted documents instead of having to submit a new application.
- In paragraph 15, at the EAEU level, regulate the deadlines for the procedure for conducting a pharmaceutical inspection from the moment the authorized body makes a decision to conduct it: within a period not exceeding 160 working days, in order to avoid delays in the inspection process and allow participants in the circulation sphere to more accurately plan the cycles for bringing the dossier into compliance with the requirements of the EAEU Rules, registration, and confirmation of registration of veterinary medicines.
- In paragraph 17, increase the period for sending the inspection program to the inspected entity: no later than 20 working days before the start of the inspection, to improve the preparation of the parties for the inspection and optimize the timing of the inspection.
- In paragraph 32, change the conditions for issuing the EAEU GMP certificate: subject to the elimination of Major non-conformities within the period specified in the CAPA plan, but not exceeding the validity period of the certificate in the event of the elimination of Critical non-conformities by the manufacturer, or in the event of the absence of Critical non-conformities by the manufacturer (OR: subject to the elimination of all Critical non-conformities by the entity in the field of circulation of veterinary medicinal products).
- Supplement with the following paragraph: the possibility of conducting inspections using remote interaction tools, since the absence of such a possibility creates additional difficulties when conducting joint inspections, which require synchronizing the participation of representatives of all Member States.
- Supplement with a paragraph on the possibility of issuing a new EAEU GMP certificate with the expiration date of the previously issued certificate, to provide the opportunity to update information (in terms of administrative changes that do not affect the actual place of manufacturing) in the current certificate without the need to conduct a repeat pharmaceutical inspection.
At the end of February, a press conference of the FSBI “VGNKI” was held at the press center of the media group “Russia Today” on the results of work for 2024 [10]. The results of the inspection activities were presented by the Advisor to the director of the FSBI “VGNKI” Danil Rudniaev. He spoke about the history of the formation of the system of inspection of manufacturers of medicines for compliance with the GMP Rules requirements and summed up the results of inspections carried out in 2024: “Today, I can say without false modesty that our inspectors are known all over the world, and they are deservedly respected.”
In March, the first educational program of the Practical School of Auditors was launched at the Eurasian Academy of Good Practices. This is an educational trajectory designed to provide specialists from pharmaceutical companies with unique knowledge and competencies in the field of audit practices and key aspects of the proper functioning of a modern pharmaceutical enterprise, considering the current requirements and recommendations of the regulatory legal acts of the EAEU. As part of the launch of the program, an introductory webinar “Good Laboratory Practice at a pharmaceutical enterprise: investigation of out of specification and sampling of raw materials” was held [11].
At the end of March, the PharmPRO community of drug manufacturers and suppliers held a free webinar “Explanation of GMP requirements for packaging materials” [12]. In particular, this event provided an opportunity to hear interpretations of requirements related to printed packaging materials, get acquainted with the practices of pharmaceutical companies, and get answers to questions. In his speech, international expert Aleksandr Aleksandrov especially noted that pharmaceutical manufacturers should shift their focus from conducting incoming inspection of printed packaging materials to auditing suppliers of these materials.
You can read more about packaging materials here: https://pharmprom.ru/vse-chto-vy-vsegda-xoteli-znat-ob-upakovke-no-boyalis-sprosit-chast-4-upakovochnye-materialy, and you can read about the audit of suppliers of printed secondary packaging materials here: https://pharmprom.ru/audit-postavshhikov-pechatnyx-vtorichnyx-upakovochnyx-materialov/.
In the first quarter, pharmaceutical inspectors of the State Institution “Belarusian State Veterinary Center” (SI “BSVC”) inspected a foreign manufacturer of veterinary medicinal products – the Spanish company Hipra for compliance with the EAEU GMP Rules requirements [13]. In accordance with the current schedule of pharmaceutical inspections published on the website of the SI “BSVC” [14], 3 inspections of veterinary medicines manufacturers whose sites are located in China are planned for April 2025.
Upcoming events and activities
On April 17, the XIV International Pharmaceutical Forum PharmPRO 2025 will be held in Moscow. This is an industry platform for communication and exchange of experience between pharmaceutical companies and market participants to improve the efficiency of each [15].
Several special sessions will be organized within the framework of the forum, including a case session “From process quality to product quality and production efficiency”. This session is planned to consider the following topics: “How to provide production with resources due to the interchangeability of personnel. Experience in implementing the “Multifunctionality” project”, “On-time product release: how the digital transformation of QMS improved indicators by more than 2 times”, “Warehouse with a license from the Ministry of Industry and Trade. Why is this so important for organizing storage processes?” and others.
On April 23-25, Moscow will host the 23rd International Exhibition “Analitika Expo 2025” – the only inter-industry exhibition of laboratory equipment and chemical reagents in Russia [16].
On April 23, as part of the exhibition, a “Forum of specialists on the quality and registration of medicinal products” will be organized with the support of the GxP Training Center. Among the announced topics: “Registration dossier: a guide for Qualified Persons”, “Inspection of dietary supplements: at the intersection of HACCP and GMP”.
Manufacturers are encouraged to take an active part in GM(D)P-related events and activities and prepare more thoroughly for GMP inspections.
The material presented was prepared using data relevant to 31.03.2025. In case of new or additional data the article can be updated.
The Federal State Budgetary Institution “The All-Russian State Center for Quality and Standardization of Veterinary Drugs and Feed”, FSBI “VGNKI” is an institution authorized by the Federal Service for Veterinary and Phytosanitary Surveillance to conduct inspections of manufacturers of medicinal products for veterinary use manufactured outside the Russian Federation for compliance with the GMP Rules requirements
The State Institution “Belarusian State Veterinary Center”, SI “BSVC” is an institution authorized by the Department of Veterinary and Food Supervision of the Ministry of Agriculture and Food of the Republic of Belarus to conduct pharmaceutical inspections of the production of veterinary medicinal products for compliance with the GMP Rules requirements
References:
- Россельхознадзор / Деятельность / Госуслуги / Выдача заключения о соответствии производителя (иностранного производителя) лекарственных средств для ветеринарного применения требованиям правил надлежащей производственной практики / Дополнительная информация для заявителя / Государственный реестр заключений о соответствии производителя (иностранного производителя) лекарственных средств для ветеринарного применения требованиям правил надлежащей производственной практики. URL: https://fsvps.gov.ru/ru/gosuslugi/zaklyuchenie-o-sootvetstvii (дата обращения 31.03.2025)
- RNC Pharma / Новости / Розничный рынок ветеринарных препаратов в России. Итоги 2024 г. и перспективы развития в 2025 г. URL: https://rncph.ru/blog/roznichnyj-rynok-veterinarnyh-preparatov-v-rossii-itogi-2024-g-i-perspektivy-razvitiya-v-2025-g/ (дата обращения 31.03.2025)
- Интерфакс / В России / Правительство до 2030 года направит на создание российских ветпрепаратов 5 млрд рублей. URL: https://www.interfax.ru/russia/1016865 (дата обращения 31.03.2025)
- ФГБУ «ВГНКИ» / Структура / Отдел инспекции производства на соответствие требованиям надлежащей производственной практики / График проведения инспектирования иностранных производителей на соответствие требованиям надлежащей производственной практики. URL: https://www.vgnki.ru/assets/2024/files/grafik-na-sajt-14032025.pdf (дата обращения 31.03.2025)
- ПРОМОМЕД / Новости / «ПРОМОМЕД» получил сертификат соответствия требованиям GMP ЕАЭС при производстве ветеринарной продукции. URL: https://promomed.ru/news/promomed-poluchil-sertifikat-sootvetstviya-trebovaniyam-gmp-eaes-pri-proizvodstve-veterinarnoy-produktsii (дата обращения 31.03.2025)
- Федеральный портал проектов нормативных правовых актов / Проект постановления Правительства Российской Федерации «Об определении соответствия производителей лекарственных средств требованиям правил надлежащей производственной практики». URL: https://regulation.gov.ru/Regulation/Npa/PublicView?npaID=154615 (дата обращения 31.03.2025)
- КонсультантПлюс / Постановление Правительства РФ от 03.12.2015 N 1314 (ред. от 05.09.2020) «Об определении соответствия производителей лекарственных средств требованиям правил надлежащей производственной практики» (вместе с «Правилами организации и проведения инспектирования производителей лекарственных средств на соответствие требованиям правил надлежащей производственной практики, а также выдачи заключений о соответствии производителя лекарственных средств указанным требованиям»). URL: https://www.consultant.ru/document/cons_doc_LAW_190256/ (дата обращения 31.03.2025)
- Евразийский экономический союз / Решение Совета Евразийской экономической комиссии от 21.01.2022 № 1 «О Правилах регулирования обращения ветеринарных лекарственных средств на таможенной территории Евразийского экономического союза. URL: https://docs.eaeunion.org/Pages/DisplayDocument.aspx?s=%7Be1f13d1d-5914-465c-835f-2aa3762eddda%7D&w=9260b414-defe-45cc-88a3-eb5c73238076&l=%7B8a412e96-924f-4b3c-8321-0d5e767e5f91%7D&EntityID=32004 (дата обращения 31.03.2025)
- Фармпром / Репортаж / Эксперты предлагают изменения в правила регулирования ветеринарных лекарств в ЕАЭС. URL: https://pharmprom.ru/eksperty-predlagayut-izmeneniya-v-pravila-regulirovaniya-veterinarnyx-lekarstv-eaes/ (дата обращения 31.03.2025)
- ФГБУ «ВГНКИ» / Пресс-центр / Новости / ФГБУ «ВГНКИ» провело пресс-конференцию по итогам работы за 2024 годи. URL: https://www.vgnki.ru/fgbu-vgnki-provelo-press-konferenciyu-po-itogam-raboty-za-2024-god.html (дата обращения 31.03.2025)
- Евразийская Академия надлежащих практик / Новости / В рамках запуска Практической школы аудиторов состоялся вводный вебинар «Надлежащая лабораторная практика на фармацевтическом предприятии: расследование отклонений от спецификаций и отбор проб исходного сырья». URL: https://gxp-academy.org/about/news_and_events/1863/ (дата обращения 31.03.2025)
- PharmPRO / Мероприятия / Разъяснение требований GMP в отношении упаковочных материалов. URL: https://pharmpro.pro/events/razyasnenie-trebovaniy-gmp-v-otnoshenii-upakovochnykh-materialov/ (дата обращения 31.03.2025)
- Евразийский экономический союз / Решение Совета Евразийской экономической комиссии от 03.11.2016 г. № 77 «Об утверждении Правил надлежащей производственной практики Евразийского экономического союза». URL: https://docs.eaeunion.org/docs/ru-ru/01411921/cncd_21112016_77 (дата обращения 31.03.2025)
- ГУ «БГВЦ» / GMP / План проведения фармацевтических инспекций на 2024-2025 годы / Текущий график проведения фармацевтических инспекций на 2025 год. URL: https://bgvc.by/wp-content/uploads/2025/03/tekushhij-grafik.png (дата обращения 31.03.2025)
- PharmPRO / XIV фармацевтический форум PharmPRO. URL: https://events.pharmpro.pro/forum-2025 (дата обращения 31.03.2025)
- Аналитика Экспо / Деловая программа / Форум специалистов по качеству и регистрации лекарственных средств. URL: https://analitikaexpo.com/ru/agenda/bp_25/SmartLab/Day1-forum/ (дата обращения 31.03.2025)

